Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea
- PMID: 27564256
- PMCID: PMC5341835
- DOI: 10.18632/oncotarget.11479
Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea
Abstract
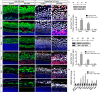
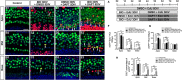
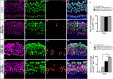
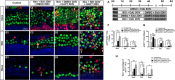
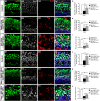
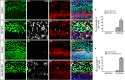
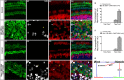
Hair cell (HC) loss is the main cause of permanent hearing loss in mammals. Previous studies have reported that in neonatal mice cochleae, Wnt activation promotes supporting cell (SC) proliferation and Notch inhibition promotes the trans-differentiation of SCs into HCs. However, Wnt activation alone fails to regenerate significant amounts of new HCs, Notch inhibition alone regenerates the HCs at the cost of exhausting the SC population, which leads to the death of the newly regenerated HCs. Mitotic HC regeneration might preserve the SC number while regenerating the HCs, which could be a better approach for long-term HC regeneration. We present a two-step gene manipulation, Wnt activation followed by Notch inhibition, to accomplish mitotic regeneration of HCs while partially preserving the SC number. We show that Wnt activation followed by Notch inhibition strongly promotes the mitotic regeneration of new HCs in both normal and neomycin-damaged cochleae while partially preserving the SC number. Lineage tracing shows that the majority of the mitotically regenerated HCs are derived specifically from the Lgr5+ progenitors with or without HC damage. Our findings suggest that the co-regulation of Wnt and Notch signaling might provide a better approach to mitotically regenerate HCs from Lgr5+ progenitor cells.
Keywords: Notch; Wnt; lineage tracing; proliferation; regeneration.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
Similar articles
-
Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):166-71. doi: 10.1073/pnas.1415901112. Epub 2014 Dec 22. Proc Natl Acad Sci U S A. 2015. PMID: 25535395 Free PMC article.
-
Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles.Sci Rep. 2016 Jul 20;6:29418. doi: 10.1038/srep29418. Sci Rep. 2016. PMID: 27435629 Free PMC article.
-
Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea.J Neurosci. 2016 Aug 17;36(33):8734-45. doi: 10.1523/JNEUROSCI.0060-16.2016. J Neurosci. 2016. PMID: 27535918 Free PMC article.
-
Role of Wnt and Notch signaling in regulating hair cell regeneration in the cochlea.Front Med. 2016 Sep;10(3):237-49. doi: 10.1007/s11684-016-0464-9. Epub 2016 Sep 7. Front Med. 2016. PMID: 27527363 Review.
-
Therapeutic Potential of Wnt and Notch Signaling and Epigenetic Regulation in Mammalian Sensory Hair Cell Regeneration.Mol Ther. 2019 May 8;27(5):904-911. doi: 10.1016/j.ymthe.2019.03.017. Epub 2019 Mar 30. Mol Ther. 2019. PMID: 30982678 Free PMC article. Review.
Cited by
-
Stem cell-based approaches: Possible route to hearing restoration?World J Stem Cells. 2020 Jun 26;12(6):422-437. doi: 10.4252/wjsc.v12.i6.422. World J Stem Cells. 2020. PMID: 32742560 Free PMC article. Review.
-
Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea.Development. 2018 Nov 27;145(23):dev166579. doi: 10.1242/dev.166579. Development. 2018. PMID: 30389848 Free PMC article.
-
The regenerative capacity of neonatal tissues.Development. 2022 Jun 15;149(12):dev199819. doi: 10.1242/dev.199819. Epub 2022 Jun 16. Development. 2022. PMID: 35708609 Free PMC article. Review.
-
LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22225-22236. doi: 10.1073/pnas.2000417117. Epub 2020 Aug 21. Proc Natl Acad Sci U S A. 2020. PMID: 32826333 Free PMC article.
-
miR‑10b‑3p, miR‑8112 and let‑7j as potential biomarkers for autoimmune inner ear diseases.Mol Med Rep. 2019 Jul;20(1):171-181. doi: 10.3892/mmr.2019.10248. Epub 2019 May 16. Mol Med Rep. 2019. PMID: 31115534 Free PMC article.
References
-
- Colin Mathers AS, Concha Marisol. Global burden of hearing loss in the year 2000. Geneva: World Health Organization; 2000. Available at http://www.whoint/healthinfo/statistics/bod_hearinglosspdf.
-
- Duthey B. Priority Medicines for Europe and the World “A Public Health Approach to Innovation” Update on 2004 Background Paper. BP 6.21 Hearing Loss. Geneva: WHOInt; 2013. Available at http://www.who.int/medicines/areas/priority_medicines/BP6_21Hearing.pdf?....
-
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. - PubMed
-
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nature medicine. 2003;9:1293–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

