Efficient Synthesis of Fully Substituted Pyrrolidine-Fused 3-Spirooxindoles via 1,3-Dipolar Cycloaddition of Aziridine and 3-Ylideneoxindole
- PMID: 27563867
- PMCID: PMC6274301
- DOI: 10.3390/molecules21091113
Efficient Synthesis of Fully Substituted Pyrrolidine-Fused 3-Spirooxindoles via 1,3-Dipolar Cycloaddition of Aziridine and 3-Ylideneoxindole
Abstract
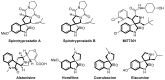
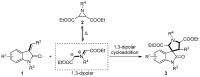
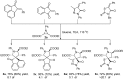
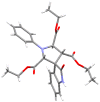
Drug-like spirocyclic scaffolds have been prepared by fusing fully functionalized pyrrolidine with oxindoles in an approach based on 1,3-dipolar cycloaddition. Reaction between aziridine and 3-ylideneoxindole generated diverse spirooxindole-pyrrolidines in good yield (up to 95%) with high diastereoselectivity (up to >20:1). The reaction also proceeded smoothly with several other synthetically useful activated trisubstituted olefins. The mild reaction conditions, short reaction times, and high tolerance for various substitutions make this approach attractive for constructing pharmacologically interesting spiro-architectures.
Keywords: 1,3-dipolar cycloaddition; aziridine; single-step reaction; spirooxindole-pyrrolidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Facile One-Pot Construction of Succinimide-Fused Spiro[Pyrrolidine-2,3'-Oxindoles] via 1,3-Dipolar Cycloaddition Involving 3-Amino Oxindoles and Maleimides.Molecules. 2018 Mar 5;23(3):582. doi: 10.3390/molecules23030582. Molecules. 2018. PMID: 29510590 Free PMC article.
-
Combinatorial synthesis of functionalized spirooxindole-pyrrolidine/pyrrolizidine/pyrrolothiazole derivatives via three-component 1,3-dipolar cycloaddition reactions.ACS Comb Sci. 2014 Sep 8;16(9):506-12. doi: 10.1021/co500085t. Epub 2014 Jul 29. ACS Comb Sci. 2014. PMID: 25033950
-
1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments.Mol Divers. 2023 Oct;27(5):2365-2397. doi: 10.1007/s11030-022-10510-9. Epub 2022 Aug 4. Mol Divers. 2023. PMID: 35925529 Review.
-
Selective C3-Allylation and Formal [3 + 2]-Annulation of Spiro-Aziridine Oxindoles: Synthesis of 5'-Substituted Spiro[pyrrolidine-3,3'-oxindoles] and Coerulescine.J Org Chem. 2022 Jul 1;87(13):8656-8671. doi: 10.1021/acs.joc.2c00863. Epub 2022 Jun 22. J Org Chem. 2022. PMID: 35731944
-
Molecular diversity of spirooxindoles. Synthesis and biological activity.Mol Divers. 2016 Feb;20(1):299-344. doi: 10.1007/s11030-015-9629-8. Epub 2015 Sep 29. Mol Divers. 2016. PMID: 26419598 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

