The immunology of post-kala-azar dermal leishmaniasis (PKDL)
- PMID: 27553063
- PMCID: PMC4995613
- DOI: 10.1186/s13071-016-1721-0
The immunology of post-kala-azar dermal leishmaniasis (PKDL)
Abstract
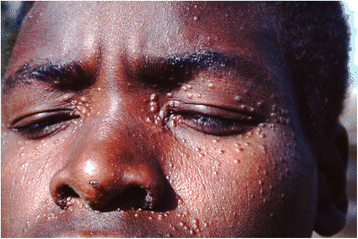
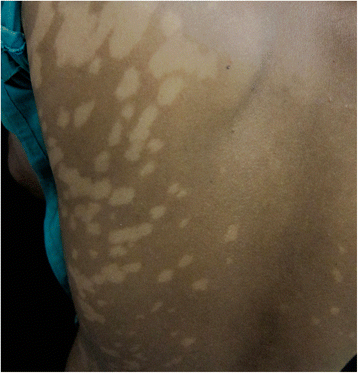
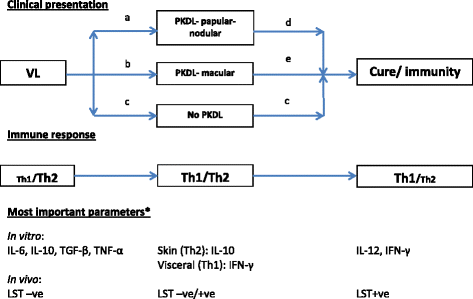
Post-kala-azar dermal leishmaniasis (PKDL) is a common complication of visceral leishmaniasis (VL) caused by Leishmania donovani. Because of its possible role in transmission it is considered a public health problem in VL endemic areas. The clinical features include a skin rash consisting of macules, papules or nodules in an otherwise healthy individual; this presentation is determined by the immune response towards parasites in the skin that probably persisted from the previous VL episode. The immune response in VL, cured VL and PKDL is the result of changes in the cytokine profile that only in part can be captured under the Th1 and Th2 dichotomy. Regulatory T cells and Th 17 cells also play a role. VL is characterized by an absent immune response to Leishmania with a predominantly Th2 type of response with high levels of IL-10; after successful treatment the patient will be immune with in vitro features of a Th1 type of response and in vivo a positive leishmanin skin test. PKDL takes an intermediate position with a dissociation of the immune response between the skin and the viscera, with a Th2 and Th1 type of response, respectively. It is likely that immune responses determine the different epidemiological and clinical characteristics of PKDL in Asia and Africa; various risk factors for PKDL may influence this, such as incomplete and inadequate treatment of VL, parasite resistance and genetic factors. It should be noted that PKDL is a heterogeneous and dynamic condition and patients differ with regard to time of onset after visceral leishmaniasis (VL), chronicity, extent and appearance of the rash including related immune responses, all of which may vary over time. Better understanding of these immune responses may offer opportunities for manipulation including combined chemotherapy and immunotherapy for VL to prevent PKDL from occurring and similarly in the treatment of chronic or treatment resistant PKDL cases.
Keywords: Clinical features; Immune manipulation; Immune responses; Immunosuppression; PKDL; Post-kala-azar dermal leishmaniasis; Visceral leishmaniasis.
Figures
Similar articles
-
PKDL and other dermal lesions in HIV co-infected patients with Leishmaniasis: review of clinical presentation in relation to immune responses.PLoS Negl Trop Dis. 2014 Nov 20;8(11):e3258. doi: 10.1371/journal.pntd.0003258. eCollection 2014. PLoS Negl Trop Dis. 2014. PMID: 25412435 Free PMC article. Review.
-
Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme.PLoS Negl Trop Dis. 2017 Nov 16;11(11):e0005877. doi: 10.1371/journal.pntd.0005877. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29145397 Free PMC article. Review.
-
IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India.J Immunol. 2007 Oct 15;179(8):5592-603. doi: 10.4049/jimmunol.179.8.5592. J Immunol. 2007. PMID: 17911647 Clinical Trial.
-
Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis.Trans R Soc Trop Med Hyg. 2001 Apr;95 Suppl 1:S59-76. doi: 10.1016/s0035-9203(01)90219-6. Trans R Soc Trop Med Hyg. 2001. PMID: 11370251
-
Post-kala-azar dermal leishmaniasis and leprosy: case report and literature review.BMC Infect Dis. 2015 Nov 23;15:543. doi: 10.1186/s12879-015-1260-x. BMC Infect Dis. 2015. PMID: 26592919 Free PMC article. Review.
Cited by
-
A randomized, double-blind phase 2b trial to evaluate efficacy of ChAd63-KH for treatment of post kala-azar dermal leishmaniasis.Mol Ther Methods Clin Dev. 2024 Jul 30;32(3):101310. doi: 10.1016/j.omtm.2024.101310. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39253357 Free PMC article.
-
VL-HIV co-infection with Leishmania containing skin lesions resembling para-kala-azar dermal leishmaniasis.PLoS Negl Trop Dis. 2024 Aug 26;18(8):e0012438. doi: 10.1371/journal.pntd.0012438. eCollection 2024 Aug. PLoS Negl Trop Dis. 2024. PMID: 39186781 Free PMC article.
-
Post kala-azar dermal leishmaniasis burden at the village level in selected high visceral leishmaniasis endemic upazilas in Bangladesh.Int J Infect Dis. 2024 Oct;147:107213. doi: 10.1016/j.ijid.2024.107213. Epub 2024 Aug 22. Int J Infect Dis. 2024. PMID: 39179149 Free PMC article.
-
Differences in the Cellular Immune Response during and after Treatment of Sudanese Patients with Post-kala-azar Dermal Leishmaniasis, and Possible Implications for Outcome.J Epidemiol Glob Health. 2024 Sep;14(3):1167-1179. doi: 10.1007/s44197-024-00270-0. Epub 2024 Jul 15. J Epidemiol Glob Health. 2024. PMID: 39007942 Free PMC article.
-
The Potential Use of Peptides in the Fight against Chagas Disease and Leishmaniasis.Pharmaceutics. 2024 Feb 4;16(2):227. doi: 10.3390/pharmaceutics16020227. Pharmaceutics. 2024. PMID: 38399281 Free PMC article. Review.
References
-
- Kemp K, Hviid L, Kharazmi A, Kemp M. Interferon-gamma production by human T cells and natural killer cells in vitro in response to antigens from the two intracellular pathogens Mycobacterium tuberculosis and Leishmania major. Scand J Immunol. 1997;46(5):495–9. doi: 10.1046/j.1365-3083.1997.d01-154.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

