Cathepsin B launches an apoptotic exit effort upon cell death-associated disruption of lysosomes
- PMID: 27551506
- PMCID: PMC4979493
- DOI: 10.1038/cddiscovery.2016.12
Cathepsin B launches an apoptotic exit effort upon cell death-associated disruption of lysosomes
Abstract
The release of cathepsin proteases from disrupted lysosomes results in lethal cellular autodigestion. Lysosomal disruption-related cell death is highly variable, showing both apoptotic and necrotic outcomes. As the substrate spectrum of lysosomal proteases encompasses the apoptosis-regulating proteins of the Bcl-2 family, their degradation could influence the cell death outcome upon lysosomal disruption. We used Förster resonance energy transfer (FRET)-based biosensors to image the real-time degradation of the Bcl-2-family members, Bcl-xl, Bax and Bid, in living cells undergoing lysosomal lysis and identified an early chain of proteolytic events, initiated by the release of cathepsin B, which directs cells toward apoptosis. In this apoptotic exit strategy, cathepsin B's proteolytic activity results in apoptosis-inducing Bid and removes apoptosis-preventing Bcl-xl. Cathepsin B furthermore appears to degrade a cystein protease that would otherwise have eliminated apoptosis-supporting Bax, indirectly keeping cellular levels of the Bax protein up. The concerted effort of these three early events shifts the balance of cell fate away from necrosis and toward apoptosis.
Figures
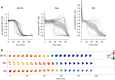
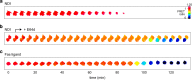
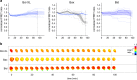
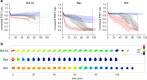
Similar articles
-
Lysosome-mediated apoptosis is associated with cathepsin D-specific processing of bid at Phe24, Trp48, and Phe183.Ann Clin Lab Sci. 2012 Summer;42(3):231-42. Ann Clin Lab Sci. 2012. PMID: 22964611
-
DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX.Cell Death Dis. 2015 Jan 29;6(1):e1624. doi: 10.1038/cddis.2014.546. Cell Death Dis. 2015. PMID: 25633293 Free PMC article.
-
Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis.Gastroenterology. 2005 Jul;129(1):269-84. doi: 10.1053/j.gastro.2005.05.022. Gastroenterology. 2005. PMID: 16012953
-
The Bcl-xL and Bax-alpha control points: modulation of apoptosis induced by cancer chemotherapy and relation to TPCK-sensitive protease and caspase activation.Biochem Cell Biol. 1997;75(4):301-14. Biochem Cell Biol. 1997. PMID: 9493953 Review.
-
Lysosomal-mitochondrial cross-talk during cell death.Mitochondrion. 2010 Nov;10(6):662-9. doi: 10.1016/j.mito.2010.07.008. Epub 2010 Aug 7. Mitochondrion. 2010. PMID: 20696281 Review.
Cited by
-
Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications.Front Oncol. 2021 Jul 26;11:654428. doi: 10.3389/fonc.2021.654428. eCollection 2021. Front Oncol. 2021. PMID: 34381705 Free PMC article. Review.
-
The relationship between vacuolation and initiation of PCD in rice (Oryza sativa) aleurone cells.Sci Rep. 2017 Jan 24;7:41245. doi: 10.1038/srep41245. Sci Rep. 2017. PMID: 28117452 Free PMC article.
-
All Roads Lead to Cathepsins: The Role of Cathepsins in Non-Alcoholic Steatohepatitis-Induced Hepatocellular Carcinoma.Biomedicines. 2022 Sep 21;10(10):2351. doi: 10.3390/biomedicines10102351. Biomedicines. 2022. PMID: 36289617 Free PMC article. Review.
-
Accessing Intracellular Targets through Nanocarrier-Mediated Cytosolic Protein Delivery.Trends Pharmacol Sci. 2020 Oct;41(10):743-754. doi: 10.1016/j.tips.2020.08.005. Epub 2020 Sep 2. Trends Pharmacol Sci. 2020. PMID: 32891429 Free PMC article. Review.
-
Norovirus-mediated translation repression promotes macrophage cell death.PLoS Pathog. 2024 Sep 3;20(9):e1012480. doi: 10.1371/journal.ppat.1012480. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39226332 Free PMC article.
References
-
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene 2004; 23: 2881–2890. - PubMed
-
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene 2008; 27: 6434–6451. - PubMed
-
- Česen MH, Pegan K, Špes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 2012; 318: 1245–1251. - PubMed
-
- de Duve C. Lysosomes, a new group of cytoplasmic particles In: Hayashi T. Subcellular Particles. The Ronald Press Co.: New York, NY, USA, 1959; 128–159.
-
- Ichinose S, Usuda J, Hirata T, Inoue T, Ohtani K, Maehara S et al. Lysosomal cathepsin initiates apoptosis, which is regulated by photodamage to Bcl-2 at mitochondria in photodynamic therapy using a novel photosensitizer, ATX-s10 (Na). Int J Oncol 2006; 29: 349–355. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

