The priming effect of previous natural pandemic H1N1 infection on the immunogenicity to subsequent 2010-2011 influenza vaccination in children: a prospective cohort study
- PMID: 27549626
- PMCID: PMC4994212
- DOI: 10.1186/s12879-016-1769-7
The priming effect of previous natural pandemic H1N1 infection on the immunogenicity to subsequent 2010-2011 influenza vaccination in children: a prospective cohort study
Abstract
Background: The effect of previous natural pandemic H1N1 (H1N1 pdm09) influenza infection on the immunogenicity to subsequent inactivated influenza vaccination in children has not been well studied. We aimed to evaluate the effect of H1N1 pdm09 natural infection and vaccination on the immunogenicity to subsequent 2010-2011 seasonal inactivated influenza vaccination in children.
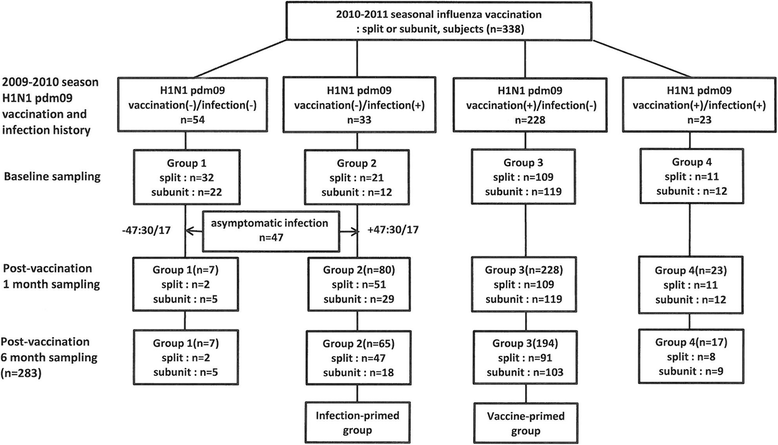
Methods: From October 2010 to May 2011, we conducted an open-label, multi-center study in children aged 6 months -18 years in Korea. We measured antibody titers with a hemagglutination-inhibition (HI) assay at baseline, 1 month, and 6 months after vaccination with trivalent split or subunit vaccines containing H1N1 pdm, A/H3N2, and B. The subjects were classified into 4 groups depending on the presence of laboratory-confirmed H1N1 pdm09 infection and/or vaccination in the 2009-2010 season; Group I: vaccination (-)/infection(-), Group II: vaccination (-)/infection(+), Group III: vaccination (+)/infection(-), Group IV: vaccination (+)/infection(+).
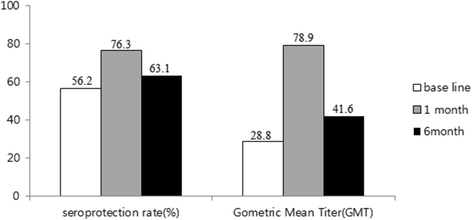
Results: Among the subjects in group I, 47 subjects who had a baseline titer >1:10 were considered to have an asymptomatic infection. They were included into the final group II (n = 80). We defined the new group II as the infection-primed (IP) group and group III as the vaccine-primed (VP) group. Seroconversion rate (57.5 % vs 35.9 %, p = 0.001), seroprotection rate at 6 months after vaccination (70.8 % vs 61.8 %, p = 0.032), and GMT at 1 month after vaccination (129.9 vs 66.5, p = 0.002) were significantly higher in the IP group than in the VP group. In the 9-18 year-old group, seroconversion rate and immunogenicity at 1 and 6 months were significantly higher in the IP group than in the VP group. However in the 1-7 year-old age group, there was no significant difference between the two groups.
Conclusions: Previous H1N1 pdm09 infection appears to have positive effects on immunogenicity of subsequent inactivated influenza vaccines against H1N1 pdm09 in older children.
Keywords: Children; Immunogenicity; Influenza vaccines; Natural pandemic H1N1 infection.
Figures
Similar articles
-
Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers.Pediatrics. 2006 Sep;118(3):e579-85. doi: 10.1542/peds.2006-0201. Pediatrics. 2006. PMID: 16950949 Clinical Trial.
-
Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming?Pediatrics. 2006 Sep;118(3):e570-8. doi: 10.1542/peds.2006-0198. Pediatrics. 2006. PMID: 16950948 Clinical Trial.
-
Immunological persistence of a seasonal influenza vaccine in people more than 3 years old.Hum Vaccin Immunother. 2015;11(7):1648-53. doi: 10.1080/21645515.2015.1037998. Hum Vaccin Immunother. 2015. PMID: 26083828 Free PMC article. Clinical Trial.
-
Key points in evaluating immunogenicity of pandemic influenza vaccines: A lesson from immunogenicity studies of influenza A(H1N1)pdm09 vaccine.Vaccine. 2017 Sep 18;35(39):5303-5308. doi: 10.1016/j.vaccine.2017.07.092. Epub 2017 Aug 4. Vaccine. 2017. PMID: 28784284 Review.
-
Comparison of the immunogenicity and safety of quadrivalent and tetravalent influenza vaccines in children and adolescents.Vaccine. 2020 Feb 5;38(6):1332-1344. doi: 10.1016/j.vaccine.2019.11.071. Epub 2020 Jan 14. Vaccine. 2020. PMID: 31948819 Review.
Cited by
-
Antiviral Approaches against Influenza Virus.Clin Microbiol Rev. 2023 Mar 23;36(1):e0004022. doi: 10.1128/cmr.00040-22. Epub 2023 Jan 16. Clin Microbiol Rev. 2023. PMID: 36645300 Free PMC article. Review.
-
Different Repeat Annual Influenza Vaccinations Improve the Antibody Response to Drifted Influenza Strains.Sci Rep. 2017 Jul 12;7(1):5258. doi: 10.1038/s41598-017-05579-4. Sci Rep. 2017. PMID: 28701762 Free PMC article.
-
Similar severity of influenza primary and re-infections in pre-school children requiring outpatient treatment due to febrile acute respiratory illness: prospective, multicentre surveillance study (2013-2015).BMC Infect Dis. 2022 Jan 4;22(1):12. doi: 10.1186/s12879-021-06988-7. BMC Infect Dis. 2022. PMID: 34983428 Free PMC article.
-
The Effects of Birth Year, Age and Sex on Hemagglutination Inhibition Antibody Responses to Influenza Vaccination.Vaccines (Basel). 2018 Jul 3;6(3):39. doi: 10.3390/vaccines6030039. Vaccines (Basel). 2018. PMID: 29970820 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical