Iron-sulfur cluster exchange reactions mediated by the human Nfu protein
- PMID: 27538573
- PMCID: PMC5167677
- DOI: 10.1007/s00775-016-1381-8
Iron-sulfur cluster exchange reactions mediated by the human Nfu protein
Abstract
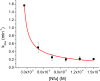
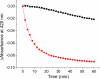
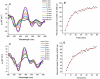
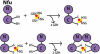
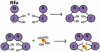
Human Nfu is an iron-sulfur cluster protein that has recently been implicated in multiple mitochondrial dysfunctional syndrome (MMDS1). The Nfu family of proteins shares a highly homologous domain that contains a conserved active site consisting of a CXXC motif. There is less functional conservation between bacterial and human Nfu proteins, particularly concerning their Iron-sulfur cluster binding and transfer roles. Herein, we characterize the cluster exchange chemistry of human Nfu and its capacity to bind and transfer a [2Fe-2S] cluster. The mechanism of cluster uptake from a physiologically relevant [2Fe-2S](GS)4 cluster complex, and extraction of the Nfu-bound iron-sulfur cluster by glutathione are described. Human holo Nfu shows a dimer-tetramer equilibrium with a protein to cluster ratio of 2:1, reflecting the Nfu-bridging [2Fe-2S] cluster. This cluster can be transferred to apo human ferredoxins at relatively fast rates, demonstrating a direct role for human Nfu in the process of [2Fe-2S] cluster trafficking and delivery.
Keywords: Cluster exchange; Iron–sulfur cluster; Metalloprotein; Mitochondrial disease; Nfu.
Figures
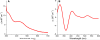
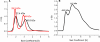
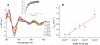
Similar articles
-
Glutathione-complexed [2Fe-2S] clusters function in Fe-S cluster storage and trafficking.J Biol Inorg Chem. 2016 Oct;21(7):887-901. doi: 10.1007/s00775-016-1387-2. Epub 2016 Sep 2. J Biol Inorg Chem. 2016. PMID: 27590019
-
Cytosolic iron-sulfur cluster transfer-a proposed kinetic pathway for reconstitution of glutaredoxin 3.FEBS Lett. 2016 Dec;590(24):4531-4540. doi: 10.1002/1873-3468.12491. Epub 2016 Dec 1. FEBS Lett. 2016. PMID: 27859051 Free PMC article.
-
[2Fe-2S] cluster transfer in iron-sulfur protein biogenesis.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6203-8. doi: 10.1073/pnas.1400102111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733926 Free PMC article.
-
Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease.Biochim Biophys Acta. 2015 Jun;1853(6):1294-315. doi: 10.1016/j.bbamcr.2014.10.014. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25448035 Review.
-
Mitochondrial [2Fe-2S] ferredoxins: new functions for old dogs.FEBS Lett. 2023 Jan;597(1):102-121. doi: 10.1002/1873-3468.14546. Epub 2022 Dec 7. FEBS Lett. 2023. PMID: 36443530 Review.
Cited by
-
Iron-sulfur cluster biosynthesis and trafficking - impact on human disease conditions.Metallomics. 2018 Jan 24;10(1):9-29. doi: 10.1039/c7mt00180k. Metallomics. 2018. PMID: 29019354 Free PMC article. Review.
-
Iron Sulfur and Molybdenum Cofactor Enzymes Regulate the Drosophila Life Cycle by Controlling Cell Metabolism.Front Physiol. 2018 Feb 14;9:50. doi: 10.3389/fphys.2018.00050. eCollection 2018. Front Physiol. 2018. PMID: 29491838 Free PMC article.
-
Cluster exchange reactivity of [2Fe-2S] cluster-bridged complexes of BOLA3 with monothiol glutaredoxins.Metallomics. 2018 Sep 19;10(9):1282-1290. doi: 10.1039/c8mt00128f. Metallomics. 2018. PMID: 30137089 Free PMC article.
-
Understanding the Molecular Basis of Multiple Mitochondrial Dysfunctions Syndrome 1 (MMDS1)-Impact of a Disease-Causing Gly208Cys Substitution on Structure and Activity of NFU1 in the Fe/S Cluster Biosynthetic Pathway.J Mol Biol. 2017 Mar 24;429(6):790-807. doi: 10.1016/j.jmb.2017.01.021. Epub 2017 Feb 1. J Mol Biol. 2017. PMID: 28161430 Free PMC article.
-
Role of protein-glutathione contacts in defining glutaredoxin-3 [2Fe-2S] cluster chirality, ligand exchange and transfer chemistry.J Biol Inorg Chem. 2017 Oct;22(7):1075-1087. doi: 10.1007/s00775-017-1485-9. Epub 2017 Aug 23. J Biol Inorg Chem. 2017. PMID: 28836015
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

