Hormonal contraceptives for contraception in overweight or obese women
- PMID: 27537097
- PMCID: PMC9063995
- DOI: 10.1002/14651858.CD008452.pub4
Hormonal contraceptives for contraception in overweight or obese women
Abstract
Background: Obesity has reached epidemic proportions around the world. Effectiveness of hormonal contraceptives may be related to metabolic changes in obesity or to greater body mass or body fat. Hormonal contraceptives include oral contraceptives (OCs), injectables, implants, hormonal intrauterine contraception (IUC), the transdermal patch, and the vaginal ring. Given the prevalence of overweight and obesity, the public health impact of any effect on contraceptive efficacy could be substantial.
Objectives: To examine the effectiveness of hormonal contraceptives in preventing pregnancy among women who are overweight or obese versus women with a lower body mass index (BMI) or weight.
Search methods: Until 4 August 2016, we searched for studies in PubMed (MEDLINE), CENTRAL, POPLINE, Web of Science, ClinicalTrials.gov, and ICTRP. We examined reference lists of pertinent articles to identify other studies. For the initial review, we wrote to investigators to find additional published or unpublished studies.
Selection criteria: All study designs were eligible. The study could have examined any type of hormonal contraceptive. Reports had to contain information on the specific contraceptive methods used. The primary outcome was pregnancy. Overweight or obese women must have been identified by an analysis cutoff for weight or BMI (kg/m(2)).
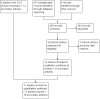
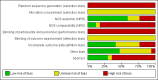
Data collection and analysis: Two authors independently extracted the data. One entered the data into RevMan and a second verified accuracy. The main comparisons were between overweight or obese women and women of lower weight or BMI. We examined the quality of evidence using the Newcastle-Ottawa Quality Assessment Scale. Where available, we included life-table rates. We also used unadjusted pregnancy rates, relative risk (RR), or rate ratio when those were the only results provided. For dichotomous variables, we computed an odds ratio with 95% confidence interval (CI).
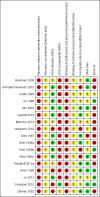
Main results: With 8 studies added in this update, 17 met our inclusion criteria and had a total of 63,813 women. We focus here on 12 studies that provided high, moderate, or low quality evidence. Most did not show a higher pregnancy risk among overweight or obese women. Of five COC studies, two found BMI to be associated with pregnancy but in different directions. With an OC containing norethindrone acetate and ethinyl estradiol (EE), pregnancy risk was higher for overweight women, i.e. with BMI ≥ 25 versus those with BMI < 25 (reported relative risk 2.49, 95% CI 1.01 to 6.13). In contrast, a trial using an OC with levonorgestrel and EE reported a Pearl Index of 0 for obese women (BMI ≥ 30) versus 5.59 for nonobese women (BMI < 30). The same trial tested a transdermal patch containing levonorgestrel and EE. Within the patch group, obese women in the "treatment-compliant" subgroup had a higher reported Pearl Index than nonobese women (4.63 versus 2.15). Of five implant studies, two that examined the six-capsule levonorgestrel implant showed differences in pregnancy by weight. One study showed higher weight was associated with higher pregnancy rate in years 6 and 7 combined (reported P < 0.05). In the other, pregnancy rates differed in year 5 among the lower weight groups only (reported P < 0.01) and did not involve women weighing 70 kg or more.Analysis of data from other contraceptive methods indicated no association of pregnancy with overweight or obesity. These included depot medroxyprogesterone acetate (subcutaneous), levonorgestrel IUC, the two-rod levonorgestrel implant, and the etonogestrel implant.
Authors' conclusions: The evidence generally did not indicate an association between higher BMI or weight and effectiveness of hormonal contraceptives. However, we found few studies for most contraceptive methods. Studies using BMI, rather than weight alone, can provide information about whether body composition is related to contraceptive effectiveness. The contraceptive methods examined here are among the most effective when used according to the recommended regimen.We considered the overall quality of evidence to be low for the objectives of this review. More recent reports provided evidence of varying quality, while the quality was generally low for older studies. For many trials the quality would be higher for their original purpose rather than the non-randomized comparisons here. Investigators should consider adjusting for potential confounding related to BMI or contraceptive effectiveness. Newer studies included a greater proportion of overweight or obese women, which helps in examining effectiveness and side effects of hormonal contraceptives within those groups.
Conflict of interest statement
Some of the trials in Grubb 1995 were conducted at FHI 360 (formerly Family Health International), where review authors are employed (LL, AB, MC, TG) or were employed (DAG, CO). None of the review authors were involved in Grubb 1995.
L Lopez. A Bernholc, T Grey, M Chen, C Otterness, FM Helmerhorst have no interests to declare.
A Edelman: Agile Pharmaceutical, consultant and served on an advisory board; Merck, trainer and served as an expert advisor; Up To Date, Inc, continuing author; FHI 360 (where review was conducted), serves on Data and Safety Monitoring Board for novel contraceptive injectable (review authors from FHI 360 are not involved in that project).
C Westhoff conducted Westhoff 2012a and studies in Table 27. She is advisor to Agile Therapeutics, which funded Kaunitz 2014, and consults with Bayer and Merck.
Figures












Update of
-
Hormonal contraceptives for contraception in overweight or obese women.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD008452. doi: 10.1002/14651858.CD008452.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Aug 18;(8):CD008452. doi: 10.1002/14651858.CD008452.pub4 PMID: 23633356 Updated. Review.
Similar articles
-
Hormonal contraceptives for contraception in overweight or obese women.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD008452. doi: 10.1002/14651858.CD008452.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Aug 18;(8):CD008452. doi: 10.1002/14651858.CD008452.pub4 PMID: 23633356 Updated. Review.
-
Hormonal contraceptives for contraception in overweight or obese women.Cochrane Database Syst Rev. 2010 Jul 7;(7):CD008452. doi: 10.1002/14651858.CD008452.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD008452. doi: 10.1002/14651858.CD008452.pub3 PMID: 20614470 Updated. Review.
-
Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus.Cochrane Database Syst Rev. 2019 Nov 12;2019(11). doi: 10.1002/14651858.CD006133.pub5. Cochrane Database Syst Rev. 2019. PMID: 31711271
-
Hormonal contraceptives for contraception in overweight or obese women.Obstet Gynecol. 2010 Nov;116(5):1206-7. doi: 10.1097/AOG.0b013e3181f81ccf. Obstet Gynecol. 2010. PMID: 20966707
-
Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus.Cochrane Database Syst Rev. 2014 Apr 30;2014(4):CD006133. doi: 10.1002/14651858.CD006133.pub5. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Nov 12;2019(11). doi: 10.1002/14651858.CD006133.pub5 PMID: 24788670 Free PMC article. Updated. Review.
Cited by
-
[Contraception in adolescents with obesity and diabetes mellitus].Probl Endokrinol (Mosk). 2022 Aug 10;68(6):137-145. doi: 10.14341/probl12760. Probl Endokrinol (Mosk). 2022. PMID: 36689719 Free PMC article. Russian.
-
Contraceptive Options Following Gestational Diabetes: Current Perspectives.Open Access J Contracept. 2019 Oct 22;10:41-53. doi: 10.2147/OAJC.S184821. eCollection 2019. Open Access J Contracept. 2019. PMID: 31749639 Free PMC article.
-
Reasons given by women for discontinuing the use of progestogen implants at Koster Hospital, North West province.S Afr Fam Pract (2004). 2022 Nov 2;64(1):e1-e7. doi: 10.4102/safp.v64i1.5471. S Afr Fam Pract (2004). 2022. PMID: 36331205 Free PMC article.
-
Women in larger bodies' experiences with contraception: a scoping review.Reprod Health. 2021 Apr 29;18(1):89. doi: 10.1186/s12978-021-01139-2. Reprod Health. 2021. PMID: 33926501 Free PMC article. Review.
-
Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: A FIGO (International Federation of Gynecology and Obstetrics) guideline.Int J Gynaecol Obstet. 2020 Sep;151 Suppl 1(Suppl 1):16-36. doi: 10.1002/ijgo.13334. Int J Gynaecol Obstet. 2020. PMID: 32894590 Free PMC article. Review. No abstract available.
References
References to studies included in this review
Burkman 2009 {published and unpublished data}
-
- Burkman RT, Fisher AC, Wan GJ, Barnowski CE, LaGuardia KD. Association between efficacy and body weight or body mass index for two low‐dose oral contraceptives. Contraception 2009;79(6):424‐7. - PubMed
-
- Hampton RM, Short M, Bieber E, Bouchard C, Ayotte N, et al. Comparison of a novel norgestimate/ethinyl estradiol oral contraceptive (Ortho Tri‐Cyclen Lo) with the oral contraceptive Loestrin Fe 1/20. Contraception 2001;63(6):289‐95. - PubMed
-
- Hampton RM, Zhang HF, Barnowski C, Wan GJ. Bleeding patterns with monophasic and triphasic low‐dose ethinyl estradiol combined oral contraceptives. Contraception 2008;77(6):415‐9. - PubMed
-
- Zhang H, LaGuardia K, Creanga D. Higher body weight and body mass index are not associated with reduced efficacy in Ortho Tri‐Cyclen Lo users (abstract). Obstetrics and Gynecology 2006;107(4 Suppl):50S.
Gemzell‐Danielson 2015 {published data only (unpublished sought but not used)}
-
- Gemzell‐Danielsson K, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low‐dose levonorgestrel intrauterine contraceptive Systems: subgroup analyses of data from a Phase III trial. PloS Pne 2015;10(9):e0135309. - PMC - PubMed
-
- Kaunitz AM, Dermout S, Tuppurainen M, Jensen J, Rosen K, Gemzell‐Danielsson K. Efficacy and safety of two low‐dose levonorgestrel intrauterine systems according to women's age: a global, multicentre, open‐label, randomized 3‐year Phase III Pearl Index study (conference poster abstract). European Journal of Contraception and Reproductive Health Care 2013;18(S1):S191‐2.
-
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low‐dose levonorgestrel intrauterine contraceptive systems. Obstetrics and Gynecology 2013;122(6):1205‐13. - PubMed
Grubb 1995 {published data only}
-
- Grubb GS, Moore D, Anderson NG. Pre‐introductory clinical trials of Norplant implants: a comparison of seventeen countries' experience. Contraception 1995;52(5):287‐96. - PubMed
Gu 1995 {published data only}
-
- Gu S, Sivin I, Du M, Zhang L, Ying L, Meng F, et al. Effectiveness of Norplant implants through seven years: a large‐scale study in China. Contraception 1995;52(2):99‐103. - PubMed
-
- Gu SJ, Du MK, Zhang LD, Liu YL, Wang SH, Sivin I. A 5‐year evaluation of NORPLANT contraceptive implants in China. Obstetrics and Gynecology 1994;83(5 Pt 1):673‐8. - PubMed
Jain 2004 {published data only}
-
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA‐SC. Contraception 2004;70(4):269‐75. - PubMed
Kaunitz 2014 {published and unpublished data}
-
- Kaunitz AM, Archer DF, Mishell DR Jr, Foegh M. Safety and tolerability of a new low‐dose contraceptive patch in obese and nonobese women. American Journal of Obstetrics and Gynecology 2015;212(3):318.e1‐8. - PubMed
-
- Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR Jr, Foegh M. Self‐reported and verified compliance in a phase 3 clinical trial of a novel low‐dose contraceptive patch and pill. Contraception 2015;91(3):204‐10. - PubMed
-
- Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR Jr, Rubin A, et al. Low‐dose levonorgestrel and ethinyl estradiol patch and pill: a randomized controlled trial. Obstetrics and Gynecology 2014;123(2 Pt 1):295‐303. - PubMed
Mansour 2012 {published data only}
-
- Mansour D, Sundstrom Poromaa I, Sommer W, Korver T. Effect of body mass index and age on the contraceptive effectiveness of nomegestrol acetate/17beta‐oestradiol and drospirenone/ethinylestradiol. European Journal of Contraception and Reproductive Health Care 2012; Vol. 17, issue Suppl 1:S95.
-
- Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β‐oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. European Journal of Contraception and Reproductive Health Care. 2011/10/15 2011; Vol. 16, issue 6:430‐43. - PMC - PubMed
Nakajima 2016 {published data only}
-
- Archer DF, Nakajima ST, Sawyer AT, Wentworth J, Trupin S, Koltun WD, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low‐dose oral contraceptive. Obstetrics and Gynecology 2013;122(3):601‐7. - PubMed
-
- Nakajima ST, Pappadakis J, Archer DF. Body mass index does not affect the efficacy or bleeding profile during use of an ultra‐low‐dose combined oral contraceptive. Contraception 2016;93(1):52‐7. - PubMed
Sivin 1997 {published data only}
-
- Sivin I, Lahteenmaki P, Ranta S, Darney P, Klaisle C, Wan L, et al. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception 1997;55(2):81‐5. - PubMed
-
- Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001;64(1):43‐9. - PubMed
Sivin 1998a {published data only}
-
- Sivin I, Alvarez F, Mishell D R Jr, Darney P, Wan L, Brache V, et al. Contraception with two levonorgestrel rod implants. A 5‐year study in the United States and Dominican Republic. Contraception 1998;58(5):275‐82. - PubMed
Sivin 1998b {published data only}
-
- Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Human Reproduction 1998;13(12):3371‐8. - PubMed
-
- Sivin I, Viegas O, Campodonico I, Diaz S, Pavez M, Wan L, et al. Clinical performance of a new two‐rod levonorgestrel contraceptive implant: a three‐year randomized study with Norplant implants as controls. Contraception 1997;55(2):73‐80. - PubMed
Sivin 1998c {published data only}
-
- Sivin I, Mishell DR Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5‐year study. Obstetrics and Gynecology 1998;92(3):337‐44. - PubMed
Westhoff 2012a {published data only}
-
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low‐dose, 91‐day, extended‐regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010;81(1):41‐8. - PubMed
-
- Westhoff CL, Hait HI, Reape KZ. Body weight does not impact pregnancy rates during use of a low‐dose extended‐regimen 91‐day oral contraceptive. Contraception. 2011/11/10 2012; Vol. 85, issue 3:235‐9. - PubMed
WHO 1990 {published data only}
-
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. I. Contraceptive efficacy and side effects. Contraception 1990;41(2):105‐24. - PubMed
-
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. II. Expulsions and removals. Contraception 1990;41(2):125‐41. - PubMed
-
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. III. The relationship between pregnancy rate and body weight. World Health Organization. Contraception 1990;41(2):143‐50. - PubMed
Xu 2012 {published data only}
Yamazaki 2015 {published data only (unpublished sought but not used)}
-
- Yamazaki M, Dwyer K, Sohban M, Davis D, Kim MJ, Soule L, et al. Effect of obesity on the effectiveness of hormonal contraceptives: an individual participant data meta‐analysis. Contraception 2015 Aug 4 [Epub ahead of print]. - PubMed
Zieman 2002 {published data only}
-
- Audet MC, Moreau M, Koltun WD, Waldbaum AS, Shangold G, Fisher AC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. Journal of the American Medical Association 2001;285(18):2347‐54. - PubMed
-
- Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. Efficacy and safety of a transdermal contraceptive system. Obstetrics and Gynecology 2001;98(5 Pt 1):799‐805. - PubMed
-
- Urdl W, Apter D, Alperstein A, Koll P, Schonian S, Bringer J, et al. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once‐weekly transdermal compared with oral contraception. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;121(2):202‐10. - PubMed
-
- Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertility and Sterility 2002;77(2 Suppl 2):S13‐8. - PubMed
References to studies excluded from this review
Banerjee 1984 {published data only}
-
- Banerjee SK, Baweja R, Bhatt RV, Chatterjee A, Choudhury SD, Coyaji B, et al. Comparative evaluation of contraceptive efficacy of norethisterone oenanthate (200 mg) injectable contraceptive given every two or three monthly. Indian Council of Medical Research Task Force on Hormonal Contraception. Contraception 1984, issue 6:561‐74. - PubMed
Berenson 2011 {published data only}
Casey 2011 {published data only}
-
- Casey PM, Long ME, Marnach ML, Bury JE. Bleeding related to etonogestrel subdermal implant in a US population. Contraception. 2011/04/12 2011; Vol. 83, issue 5:426‐30. [1879‐0518: (Electronic)] - PubMed
Cirkel 1990 {published data only}
-
- Cirkel U, Schneider HPG. [Incidence of side effects caused by a three‐stage preparation administered to 10,034 women] [[Nebenwirkungsinzidenz eines Dreistufenpräparates unter der Anwendung bei 10.034 Frauen]]. Geburtshilfe und Frauenheilkunde 1991;50(12):969‐73. - PubMed
Du 2000 {published data only}
-
- Du MK, Chow LP, Zheng HM, Chen CH. A 10‐year follow‐up study of contraceptive Norplant implants. International Journal of Gynaecology & Obstetrics. 2000/03/04 2000; Vol. 68, issue 3:249‐56. - PubMed
-
- Du MK, Zheng HM, Chen HC, Chow LP. Study of Norplant implants in Shanghai: three‐year experience. International Journal of Gynaecology & Obstetrics. 1990/12/01 1990; Vol. 33, issue 4:345‐57. - PubMed
Gemzell‐Danielsson 2015b {published data only}
-
- Gemzell‐Danielsson K, Kardos L, Hertzen H. Impact of bodyweight/body mass index on the effectiveness of emergency contraception with levonorgestrel: a pooled‐analysis of three randomized controlled trials. Current Medical Research and Opinion 2015;31(12):2241‐8. - PubMed
Graesslin 2008 {published data only}
-
- Graesslin O, Korver T. The contraceptive efficacy of Implanon®: a review of clinical trials and marketing experience. European Journal of Contraception and Reproductive Health Care 2008;13(Suppl 1):4‐12. - PubMed
Kapp 2015 {published data only}
-
- Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011/09/17 2011; Vol. 84, issue 4:363‐7. - PubMed
-
- Kapp N, Abitbol JL, Mathe H, Scherrer B, Guillard H, Gainer E, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception 2015;91(2):97‐104. - PubMed
McNicholas 2013 {published data only (unpublished sought but not used)}
Moreau 2012 {published data only}
Mornar 2012 {published data only}
-
- Mornar S, Chan L‐N, Mistretta S, Neustadt A, Martins S, Gilliam M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. American Journal of Obstetrics and Gynecology August 2012; Vol. 207, issue 110:e1‐6. [NCT00724438] - PubMed
-
- Neustadt A, Gilliam M. Pharmacokinetics of Implanon in obese women. http://clinicaltrials.gov/ct2/show/NCT00724438 (accessed 15 Sep 2009). [NCT00724438]
Sivin 1988 {published data only}
-
- Sivin I. International experience with NORPLANT® and NORPLANT®‐2 contraceptives. Studies in Family Planning 1988;19(2):81‐94. - PubMed
Sivin 2000 {published data only}
-
- Sivin I, Mishell DR, Diaz S, Biswas A, Alvarez F, Darney P, et al. Prolonged effectiveness of Norplant(R) capsule implants: a 7‐year study. Contraception 2000, issue 3:187‐94. - PubMed
Weisberg 1999 {published data only}
-
- Weisberg E, Fraser IS, Lacarra M, Mishell DR, Jr, Alvarez F, et al. Efficacy, bleeding patterns, and side effects of a 1‐year contraceptive vaginal ring. Contraception 1999;59(5):311‐8. - PubMed
Additional references
ASEC 2015
-
- American Society for Emergency Contraception. Efficacy of emergency contraception and body weight: Current understanding and recommendations. http://americansocietyforec.org/uploads/3/2/7/0/3270267/asec_ec_efficacy.... [Princeton, New Jersey], American Society for Emergency Contraception [ASEC], 2015 Jan., January 2015 (accessed 12 April 2016).
Brunner Huber 2006
-
- Brunner Huber LR, Hogue CJ, Stein AD, Drews C, Zieman M. Body mass index and risk for oral contraceptive failure: a case‐cohort study in South Carolina. Annals of Epidemiology 2006;16(8):637‐43. - PubMed
Brunner 2005
-
- Brunner LR, Hogue CJ. The role of body weight in oral contraceptive failure: results from the 1995 National Survey of Family Growth. Annals of Epidemiology 2005;15(7):492‐9. - PubMed
Brunner Huber 2007
-
- Brunner Huber LR, Toth JL. Obesity and oral contraceptive failure: findings from the 2002 National Survey of Family Growth. American Journal of Epidemiology 2007;166(11):1306‐11. - PubMed
Callegari 2014
-
- Callegari LS, Nelson KM, Arterburn DE, Prager SW, Schiff MA, Schwarz EB. Factors associated with lack of effective contraception among obese women in the United States. Contraception 2014;90(3):265‐71. - PubMed
CDC 2016
-
- Centers for Disease Control and Prevention. Defining Adult Overweight and Obesity. www.cdc.gov/obesity/adult/defining.html (accessed 12 May 2016).
Creinin 2015
-
- Creinin MD, Baker JB, Eisenberg L, Ginde S, Turok DK, Westhoff CL. Levonorgestrel levels in nonobese and obese women using LNG20, a new intrauterine contraceptive. Obstetrics and Gynecology 2015;125 Suppl 1:84S‐5S.
Dinger 2009
-
- Dinger JC, Cronin M, Mohner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. American Journal of Obstetrics and Gynecology 2009;201(3):263.e1‐9. - PubMed
Dinger 2011
-
- Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstetrics and Gynecology. 2011/01/11 2011; Vol. 117, issue 1:33‐40. - PubMed
ECEC 2015
-
- European Consortium for Emergency Contraception. Efficacy of emergency contraception and body weight. Current understanding and recommendations. www.ec‐ec.org/custom‐content/uploads/2015/12/ECEC‐EC‐Body‐Weight‐November‐2015.pdf November 2015 (accessed 12 Aprl 2016).
Edelman 2009
Edelman 2014
Gallo 2014
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [updated October 2013]. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook... (accessed 29 December 2015).
Grimes 2005
-
- Grimes DA, Shields WC. Family planning for obese women: challenges and opportunities. Contraception 2005;72(1):1‐4. - PubMed
Grimes 2010
-
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women's health research: science, protoscience, and pseudoscience. Fertility and Sterility 2010;93(6):1731‐4. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 26 Mar 2012).
Holt 2002
-
- Holt VL, Cushing‐Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstetrics and Gynecology 2002;99(5 Pt 1):820‐7. - PubMed
Holt 2005
-
- Holt VL, Scholes D, Wicklund KG, Cushing‐Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstetrics and Gynecology 2005;105(1):46‐52. - PubMed
Jatlaoui 2016
Kavanaugh 2015
Kohn 2015
-
- Kohn JE, Lopez PM, Simons HR. Weight and body mass index among female contraceptive clients. Contraception 2015;91(6):470‐3. - PubMed
Lopez 2013
Lopez 2014
McKeating 2015
-
- McKeating A, O'Higgins A, Turner C, McMahon L, Sheehan SR, Turner MJ. The relationship between unplanned pregnancy and maternal body mass index 2009‐2012. European Journal of Contraception and Reproductive Health Care 2015;20(6):409‐18. - PubMed
Merki‐Feld 2015
-
- Merki‐Feld GS, Skouby S, Serfaty D, Lech M, Bitzer J, Crosignani PG, et al. European society of contraception statement on contraception in obese women. European Journal of Contraception and Reproductive Health Care 2015;20(1):19‐28. - PubMed
Morrell 2016
-
- Morrell KM, Cremers S, Westhoff CL, Davis AR. Relationship between etonogestrel level and BMI in women using the contraceptive implant for more than 1 year. Contraception 2016;93(3):263‐5. [PUBMED: 26577754] - PubMed
Ng 2014
Ogden 2014
Segall‐Gutierrez 2010
-
- Segall‐Gutierrez P, Taylor D, Liu X, Stanczyk F, Azen S, Mishell DR. Follicular development and ovulation in extremely obese women receiving depo‐medroxyprogesterone acetate subcutaneously. Contraception 2010;81(6):487‐95. - PubMed
Sivin 2001
-
- Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001;64(1):43‐9. - PubMed
Strauss 2005
-
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005.
Trussell 2009
UN 2015
-
- United Nations, Department of Economic and Social Affairs. World Contraceptive Patterns 2015. www.un.org/en/development/desa/population/publications/ (accessed 23 February 2015).
Vessey 2001
-
- Vessey M. Oral contraceptive failures and body weight: findings in a large cohort study. Journal of Family Planning and Reproductive Health Care 2001;27(2):90‐1. - PubMed
Wells 2014
-
- GA Wells, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6 October 2015).
Westhoff 2005
-
- Westhoff C. Higher body weight does not affect NuvaRing's efficacy (abstract). Obstetrics and Gynecology 2005;105(4 Suppl):56S.
Westhoff 2008
-
- Westhoff C. Subject weight and oral contraceptive efficacy in recent clinical trials (abstract). Contraception 2008;78(2):167.
Westhoff 2009
-
- Westhoff C, Torgal A, Mayeda ER, Lerner J, Paik M. Ovarian suppression during oral contraceptive use in normal‐weight and obese women. Contraception 2009;80(2):210. - PubMed
Westhoff 2012b
Westhoff 2012c
-
- Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception 2012;85(5):465‐9. - PubMed
Westhoff 2014
-
- Westhoff CL, Reinecke I, Bangerter K, Merz M. Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55‐mg ethinyl estradiol/2.1‐mg gestodene: a multicenter, open‐label, uncontrolled study over three treatment cycles. Contraception 2014;90(3):272‐9. - PubMed
WHO 2012
-
- World Health Organization. Obesity. http://www.euro.who.int/en/what‐we‐do/health‐topics/noncommunicable‐dise... (accessed 27 Dec 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

