Co-inhibition of pol θ and HR genes efficiently synergize with cisplatin to suppress cisplatin-resistant lung cancer cells survival
- PMID: 27533083
- PMCID: PMC5323145
- DOI: 10.18632/oncotarget.11214
Co-inhibition of pol θ and HR genes efficiently synergize with cisplatin to suppress cisplatin-resistant lung cancer cells survival
Abstract
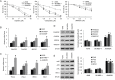
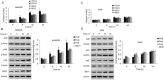
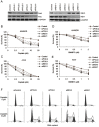
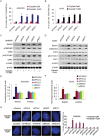
Cisplatin exert its anticancer effect by creating intrastrand and interstrand DNA cross-links which block DNA replication and is a major drug used to treat lung cancer. However, the main obstacle of the efficacy of treatment is drug resistance. Here, we show that expression of translesion synthesis (TLS) polymerase Q (POLQ) was significantly elevated by exposure of lung cancer cells A549/DR (a cisplatin-resistant A549 cell line) to cisplatin. POLQ expression correlated inversely with homologous recombination (HR) activity. Co-depletion of BRCA2 and POLQ by siRNA markedly increased sensitivity of A549/DR cells to cisplatin, which was accompanied with impairment of double strand breaks (DSBs) repair reflected by prominent cell cycle checkpoint response, increased chromosomal aberrations and persistent colocalization of p-ATM and 53BP1 foci induced by cisplatin. Thus, co-knockdown of POLQ and HR can efficiently synergize with cisplatin to inhibit A549/DR cell survival by inhibiting DNA DSBs repair. Similar results were observed in A549/DR cells co-depleted of BRCA2 and POLQ following BMN673 (a PARP inhibitor) treatment. Importantly, the sensitization effects to cisplatin and BMN673 in A549/DR cells by co-depleting BRCA2 and POLQ was stronger than those by co-depleting BRCA2 and other TLS factors including POLH, REV3, or REV1. Our results indicate that there is a synthetic lethal relationship between pol θ-mediated DNA repair and HR pathways. Pol θ may be considered as a novel target for lung cancer therapy.
Keywords: cisplatin-resistance; homologous recombination; lung caner cells; pol θ; translesion synthesis.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

Similar articles
-
Co-inhibition of Pol η and ATR sensitizes cisplatin-resistant non-small cell lung cancer cells to cisplatin by impeding DNA damage repair.Acta Pharmacol Sin. 2018 Aug;39(8):1359-1372. doi: 10.1038/aps.2017.187. Epub 2018 May 31. Acta Pharmacol Sin. 2018. PMID: 29849128 Free PMC article.
-
Knockdown of REV3 synergizes with ATR inhibition to promote apoptosis induced by cisplatin in lung cancer cells.J Cell Physiol. 2017 Dec;232(12):3433-3443. doi: 10.1002/jcp.25792. Epub 2017 Feb 13. J Cell Physiol. 2017. PMID: 28075014
-
The functional status of DNA repair pathways determines the sensitization effect to cisplatin in non-small cell lung cancer cells.Cell Oncol (Dordr). 2016 Dec;39(6):511-522. doi: 10.1007/s13402-016-0291-7. Epub 2016 Jul 29. Cell Oncol (Dordr). 2016. PMID: 27473273
-
Targeting the DNA Repair Enzyme Polymerase θ in Cancer Therapy.Trends Cancer. 2021 Feb;7(2):98-111. doi: 10.1016/j.trecan.2020.09.007. Epub 2020 Oct 24. Trends Cancer. 2021. PMID: 33109489 Review.
-
The role of DNA repair pathways in cisplatin resistant lung cancer.Cancer Treat Rev. 2014 Dec;40(10):1161-70. doi: 10.1016/j.ctrv.2014.10.003. Epub 2014 Oct 18. Cancer Treat Rev. 2014. PMID: 25458603 Review.
Cited by
-
Identification of RP-6685, an Orally Bioavailable Compound that Inhibits the DNA Polymerase Activity of Polθ.J Med Chem. 2022 Oct 13;65(19):13198-13215. doi: 10.1021/acs.jmedchem.2c00998. Epub 2022 Sep 20. J Med Chem. 2022. PMID: 36126059 Free PMC article.
-
Discovery of a small-molecule inhibitor that traps Polθ on DNA and synergizes with PARP inhibitors.Nat Commun. 2024 Apr 5;15(1):2862. doi: 10.1038/s41467-024-46593-1. Nat Commun. 2024. PMID: 38580648 Free PMC article.
-
Suppression of the FA pathway combined with CHK1 inhibitor hypersensitize lung cancer cells to gemcitabine.Sci Rep. 2017 Nov 8;7(1):15031. doi: 10.1038/s41598-017-15172-4. Sci Rep. 2017. PMID: 29118324 Free PMC article.
-
Securing the Payload, Finding the Cell, and Avoiding the Endosome: Peptide-Targeted, Fusogenic Porous Silicon Nanoparticles for Delivery of siRNA.Adv Mater. 2019 Aug;31(35):e1902952. doi: 10.1002/adma.201902952. Epub 2019 Jul 3. Adv Mater. 2019. PMID: 31267590 Free PMC article.
-
Targeting the DNA damage response in cancer.MedComm (2020). 2024 Oct 31;5(11):e788. doi: 10.1002/mco2.788. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492835 Free PMC article. Review.
References
-
- Fuertes MA, Castilla J, Alons C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Cur Med Chem. 2003;10:257–266. - PubMed
-
- Ahmad S. Platinum-DNA interactions and subsequent cellular processes controlling sensitivity to anticancer platinum complexes. Chem Biodivers. 2010;7:543–566. - PubMed
-
- Jung Y, Lippord SJ. Direct cellular response to platinum-induced DNA damage. Chem Rev. 2007;107:1387–1407. - PubMed
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Discov. 2005;4:307–320. - PubMed
-
- Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

