Quality Control of Photosystem II: The Mechanisms for Avoidance and Tolerance of Light and Heat Stresses are Closely Linked to Membrane Fluidity of the Thylakoids
- PMID: 27532009
- PMCID: PMC4969305
- DOI: 10.3389/fpls.2016.01136
Quality Control of Photosystem II: The Mechanisms for Avoidance and Tolerance of Light and Heat Stresses are Closely Linked to Membrane Fluidity of the Thylakoids
Abstract
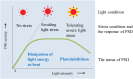
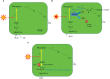
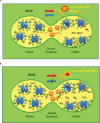
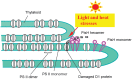
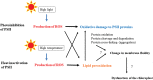
When oxygenic photosynthetic organisms are exposed to excessive light and/or heat, Photosystem II is damaged and electron transport is blocked. In these events, reactive oxygen species, endogenous radicals and lipid peroxidation products generated by photochemical reaction and/or heat cause the damage. Regarding light stress, plants first dissipate excessive light energy captured by light-harvesting chlorophyll protein complexes as heat to avoid the hazards, but once light stress is unavoidable, they tolerate the stress by concentrating damage in a particular protein in photosystem II, i.e., the reaction-center binding D1 protein of Photosystem II. The damaged D1 is removed by specific proteases and replaced with a new copy produced through de novo synthesis (reversible photoinhibition). When light intensity becomes extremely high, irreversible aggregation of D1 occurs and thereby D1 turnover is prevented. Once the aggregated products accumulate in Photosystem II complexes, removal of them by proteases is difficult, and irreversible inhibition of Photosystem II takes place (irreversible photoinhibition). Important is that various aspects of both the reversible and irreversible photoinhibition are highly dependent on the membrane fluidity of the thylakoids. Heat stress-induced inactivation of photosystem II is an irreversible process, which may be also affected by the fluidity of the thylakoid membranes. Here I describe why the membrane fluidity is a key to regulate the avoidance and tolerance of Photosystem II on environmental stresses.
Keywords: D1 protein; heat stress; light stress; lipid peroxidation; membrane fluidity; photosystem II; protein aggregation; thylakoid.
Figures

Similar articles
-
Quality control of Photosystem II: reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination.Front Plant Sci. 2013 Oct 29;4:433. doi: 10.3389/fpls.2013.00433. eCollection 2013. Front Plant Sci. 2013. PMID: 24194743 Free PMC article.
-
Assay of photoinhibition and heat inhibition of photosystem II in higher plants.Methods Mol Biol. 2011;684:201-15. doi: 10.1007/978-1-60761-925-3_17. Methods Mol Biol. 2011. PMID: 20960132
-
Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination.PLoS One. 2012;7(12):e52100. doi: 10.1371/journal.pone.0052100. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300595 Free PMC article.
-
Quality control of photosystem II.Plant Cell Physiol. 2001 Feb;42(2):121-8. doi: 10.1093/pcp/pce022. Plant Cell Physiol. 2001. PMID: 11230565 Review.
-
Quality control of Photosystem II: the molecular basis for the action of FtsH protease and the dynamics of the thylakoid membranes.J Photochem Photobiol B. 2014 Aug;137:100-6. doi: 10.1016/j.jphotobiol.2014.02.012. Epub 2014 Mar 4. J Photochem Photobiol B. 2014. PMID: 24725639 Review.
Cited by
-
High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings.Plants (Basel). 2022 May 30;11(11):1454. doi: 10.3390/plants11111454. Plants (Basel). 2022. PMID: 35684228 Free PMC article.
-
Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants.Photosynth Res. 2021 Mar;147(3):301-315. doi: 10.1007/s11120-020-00810-2. Epub 2021 Jan 4. Photosynth Res. 2021. PMID: 33394352
-
Identification of candidate regulators of the response to early heat stress in climate-adapted wheat landraces via transcriptomic and co-expression network analyses.Front Plant Sci. 2024 Jan 3;14:1252885. doi: 10.3389/fpls.2023.1252885. eCollection 2023. Front Plant Sci. 2024. PMID: 38235195 Free PMC article.
-
S2P2-the chloroplast-located intramembrane protease and its impact on the stoichiometry and functioning of the photosynthetic apparatus of A. thaliana.Front Plant Sci. 2024 Mar 15;15:1372318. doi: 10.3389/fpls.2024.1372318. eCollection 2024. Front Plant Sci. 2024. PMID: 38559762 Free PMC article.
-
Temperature-dependent regulation of electron transport and ATP synthesis in chloroplasts in vitro and in silico.Photosynth Res. 2020 Dec;146(1-3):299-329. doi: 10.1007/s11120-020-00777-0. Epub 2020 Aug 11. Photosynth Res. 2020. PMID: 32780309
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

