Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study
- PMID: 27531725
- PMCID: PMC5013416
- DOI: 10.1136/bmjopen-2015-010983
Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study
Abstract
Objectives: There is no consensus on whether studies with no observed events in the treatment and control arms, the so-called both-armed zero-event studies, should be included in a meta-analysis of randomised controlled trials (RCTs). Current analytic approaches handled them differently depending on the choice of effect measures and authors' discretion. Our objective is to evaluate the impact of including or excluding both-armed zero-event (BA0E) studies in meta-analysis of RCTs with rare outcome events through a simulation study.
Method: We simulated 2500 data sets for different scenarios varying the parameters of baseline event rate, treatment effect and number of patients in each trial, and between-study variance. We evaluated the performance of commonly used pooling methods in classical meta-analysis-namely, Peto, Mantel-Haenszel with fixed-effects and random-effects models, and inverse variance method with fixed-effects and random-effects models-using bias, root mean square error, length of 95% CI and coverage.
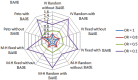
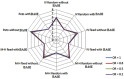
Results: The overall performance of the approaches of including or excluding BA0E studies in meta-analysis varied according to the magnitude of true treatment effect. Including BA0E studies introduced very little bias, decreased mean square error, narrowed the 95% CI and increased the coverage when no true treatment effect existed. However, when a true treatment effect existed, the estimates from the approach of excluding BA0E studies led to smaller bias than including them. Among all evaluated methods, the Peto method excluding BA0E studies gave the least biased results when a true treatment effect existed.
Conclusions: We recommend including BA0E studies when treatment effects are unlikely, but excluding them when there is a decisive treatment effect. Providing results of including and excluding BA0E studies to assess the robustness of the pooled estimated effect is a sensible way to communicate the results of a meta-analysis when the treatment effects are unclear.
Keywords: both-armed zero-event; meta-analysis; rare event outcome; simulation.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Real-world Performance of Meta-analysis Methods for Double-Zero-Event Studies with Dichotomous Outcomes Using the Cochrane Database of Systematic Reviews.J Gen Intern Med. 2019 Jun;34(6):960-968. doi: 10.1007/s11606-019-04925-8. Epub 2019 Mar 18. J Gen Intern Med. 2019. PMID: 30887438 Free PMC article.
-
What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data.Stat Med. 2004 May 15;23(9):1351-75. doi: 10.1002/sim.1761. Stat Med. 2004. PMID: 15116347
-
Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events.Stat Med. 2007 Jan 15;26(1):53-77. doi: 10.1002/sim.2528. Stat Med. 2007. PMID: 16596572
-
Aspirin Use in Adults: Cancer, All-Cause Mortality, and Harms: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2015 Sep. Report No.: 13-05193-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015 Sep. Report No.: 13-05193-EF-1. PMID: 26491756 Free Books & Documents. Review.
-
Simulation-Based Comparison of Methods for Meta-Analysis of Proportions and Rates [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 13(14)-EHC084-EF. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 13(14)-EHC084-EF. PMID: 24404633 Free Books & Documents. Review.
Cited by
-
Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis.Clin Microbiol Infect. 2023 May;29(5):578-586. doi: 10.1016/j.cmi.2023.01.010. Epub 2023 Jan 16. Clin Microbiol Infect. 2023. PMID: 36657488 Free PMC article. Review.
-
Real-world Performance of Meta-analysis Methods for Double-Zero-Event Studies with Dichotomous Outcomes Using the Cochrane Database of Systematic Reviews.J Gen Intern Med. 2019 Jun;34(6):960-968. doi: 10.1007/s11606-019-04925-8. Epub 2019 Mar 18. J Gen Intern Med. 2019. PMID: 30887438 Free PMC article.
-
Multidrug-resistant tuberculosis treatment adherence in migrants: a systematic review and meta-analysis.BMC Med. 2018 Feb 22;16(1):27. doi: 10.1186/s12916-017-1001-7. BMC Med. 2018. PMID: 29466983 Free PMC article. Review.
-
Efficacy of induction regimens for cryptococcal meningitis in HIV-infected adults: a systematic review and network meta-analysis.Sci Rep. 2021 Apr 21;11(1):8565. doi: 10.1038/s41598-021-87726-6. Sci Rep. 2021. PMID: 33883566 Free PMC article.
-
Fascia defect closure versus non-closure in minimal invasive direct inguinal hernia mesh repair: a systematic review and meta-analysis of real-world evidence.Hernia. 2023 Apr;27(2):459-469. doi: 10.1007/s10029-022-02732-5. Epub 2022 Dec 28. Hernia. 2023. PMID: 36576667
References
-
- Cochrane Group. Cochrane handbook: meta-analysis of dichotomous outcomes. http://handbook.cochrane.org/chapter_9/9_4_4_meta_analysis_of_dichotomou...
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources