Changes in Lymphangiogenesis and Vascular Endothelial Growth Factor Expression by Neo-Adjuvant Hormonal Therapy in Prostate Cancer Patients
- PMID: 27527525
- PMCID: PMC5260425
- DOI: 10.1002/pros.23244
Changes in Lymphangiogenesis and Vascular Endothelial Growth Factor Expression by Neo-Adjuvant Hormonal Therapy in Prostate Cancer Patients
Abstract
Background: The anti-cancer mechanism of neo-adjuvant hormonal therapy (NHT) is not well understood. Lymphangiogenesis plays an important role in cancer progression and is regulated by a complex mechanism that includes vascular endothelial growth factor (VEGF) signaling. However, there is little information regarding relationship between lymphangiogenesis and androgen deprivation. The aim of this study was to clarify changes in lymphangiogenesis and VEGF expression induced by androgen deprivation in prostate cancer in vivo and in vitro.
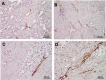
Methods: Patients who had undergone a radical prostatectomy were enrolled in the study (NHT, n = 60 and non-NHT, n = 64). Lymph vessels were identified by D2-40 immunoreactivity and lymph vessel density and lymph vessel area (LVD and LVA, respectively) were measured from micrographs. The expression of VEGF-A, -B, -C, and -D was evaluated by immunohistochemistry. The prognostic value of LVD and LVA for biochemical recurrence was also investigated.
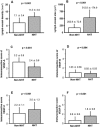
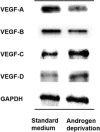
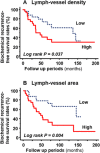
Results: Mean LVD ± SD was higher in the NHT than in the non-NHT group (11.3 ± 3.0 vs. 7.1 ± 3.4 per high power field; P < 0.001). LVA was larger in the NHT than in the non-NHT group (512.8 ± 174.9 vs. 202.7 ± 72.8 µm2 ; P < 0.001). VEGF-A expression was lower whereas VEGF-C and -D levels were higher in the NHT than in the non-NHT group. VEGF-B expression in specimens with NHT was lower than that in biopsy specimens at diagnosis. These results were confirmed by in vitro studies used androgen-sensitive prostate cancer cell line. LVA was found to be an independent predictor of biochemical recurrence in patients who received NHT.
Conclusions: Our results demonstrate that NHT stimulates lymphangiogenesis via upregulation of VEGF-C and -D, which may increase LVA and affect the outcome of prostate cancer patients. This findings were supported by in vitro data of prostate cancer cell. Prostate 77:255-262, 2017. © 2016 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Keywords: androgen deprivation; lymphangiogenesis; neo-adjuvant hormonal therapy; prostate cancer; vascular endothelial growth factors.
© 2016 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Figures
Similar articles
-
Neoadjuvant hormonal therapy for low-risk prostate cancer induces biochemical recurrence after radical prostatectomy via increased lymphangiogenesis-related parameters.Prostate. 2017 Oct;77(14):1408-1415. doi: 10.1002/pros.23402. Epub 2017 Aug 28. Prostate. 2017. PMID: 28845514 Free PMC article.
-
Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy.Prostate. 2015 Jan;75(1):84-91. doi: 10.1002/pros.22894. Epub 2014 Oct 13. Prostate. 2015. PMID: 25307287 Free PMC article.
-
Expression of insulin-like growth factor binding protein-3 before and after neoadjuvant hormonal therapy in human prostate cancer tissues: correlation with histopathologic effects and biochemical recurrence.Urology. 2004 Jun;63(6):1184-90. doi: 10.1016/j.urology.2004.02.015. Urology. 2004. PMID: 15183987
-
Neoadjuvant hormonal therapy in carcinoma of the prostate.BJU Int. 1999 Mar;83(4):438-48. doi: 10.1046/j.1464-410x.1999.00953.x. BJU Int. 1999. PMID: 10210568 Review.
-
Medical therapy of prostate cancer. A review.Minerva Urol Nefrol. 2005 Jun;57(2):71-84. Minerva Urol Nefrol. 2005. PMID: 15951731 Review.
Cited by
-
KLK3 in the Regulation of Angiogenesis-Tumorigenic or Not?Int J Mol Sci. 2021 Dec 17;22(24):13545. doi: 10.3390/ijms222413545. Int J Mol Sci. 2021. PMID: 34948344 Free PMC article. Review.
-
Neoadjuvant hormonal therapy for low-risk prostate cancer induces biochemical recurrence after radical prostatectomy via increased lymphangiogenesis-related parameters.Prostate. 2017 Oct;77(14):1408-1415. doi: 10.1002/pros.23402. Epub 2017 Aug 28. Prostate. 2017. PMID: 28845514 Free PMC article.
References
-
- Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol 2006; 15:117–128. - PubMed
-
- Miyata Y, Kanda S, Mitsunari K, Asai A, Sakai H. Heme oxygenase‐1 expression is associated with tumor aggressiveness and outcomes in patients with bladder cancer: A correlation with smoking intensity. Transl Res 2014; 164:468–476. - PubMed
-
- Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002; 296:1883–1886. - PubMed
-
- Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor‐secreted vascular endothelial growth factor‐C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 2005; 65:9789–9798. - PubMed
-
- de Brot S, Ntekim A, Cardenas R, James V, Allegrucci C, Heery DM, Bates DO, Ødum N, Persson JL, Mongan NP. Regulation of vascular endothelial growth factor in prostate cancer. Endocr Relat Cancer 2015; 22:107–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

