A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease
- PMID: 27524239
- PMCID: PMC5343756
- DOI: 10.1016/j.bbrc.2016.08.067
A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease
Abstract
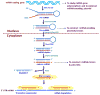
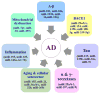
Currently, 5.4 million Americans suffer from AD, and these numbers are expected to increase up to 16 million by 2050. Despite tremendous research efforts, we still do not have drugs or agents that can delay, or prevent AD and its progression, and we still do not have early detectable biomarkers for AD. Multiple cellular changes have been implicated in AD, including synaptic damage, mitochondrial damage, production and accumulation of Aβ and phosphorylated tau, inflammatory response, deficits in neurotransmitters, deregulation of the cell cycle, and hormonal imbalance. Research into AD has revealed that miRNAs are involved in each of these cellular changes and interfere with gene regulation and translation. Recent discoveries in molecular biology have also revealed that microRNAs play a major role in post-translational regulation of gene expression. The purpose of this article is to review research that has assessed neuroprotective and neurodegenerative characteristics of microRNAs in brain samples from AD transgenic mouse models and patients with AD.
Keywords: Alzheimer's disease; Mitochondrial dysfunction; Phosphorylated tau; Synaptic damage; microRNAs.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
MicroRNAs, Aging, Cellular Senescence, and Alzheimer's Disease.Prog Mol Biol Transl Sci. 2017;146:127-171. doi: 10.1016/bs.pmbts.2016.12.009. Epub 2017 Feb 2. Prog Mol Biol Transl Sci. 2017. PMID: 28253983 Review.
-
Defective mitophagy and synaptic degeneration in Alzheimer's disease: Focus on aging, mitochondria and synapse.Free Radic Biol Med. 2021 Aug 20;172:652-667. doi: 10.1016/j.freeradbiomed.2021.07.013. Epub 2021 Jul 8. Free Radic Biol Med. 2021. PMID: 34246776 Review.
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
-
MicroRNAs Dysregulation and Mitochondrial Dysfunction in Neurodegenerative Diseases.Int J Mol Sci. 2020 Aug 20;21(17):5986. doi: 10.3390/ijms21175986. Int J Mol Sci. 2020. PMID: 32825273 Free PMC article. Review.
-
MicroRNAs (miRNAs) in neurodegenerative diseases.Brain Pathol. 2008 Jan;18(1):130-8. doi: 10.1111/j.1750-3639.2007.00120.x. Brain Pathol. 2008. PMID: 18226108 Free PMC article. Review.
Cited by
-
MicroRNA-338-5p alleviates neuronal apoptosis via directly targeting BCL2L11 in APP/PS1 mice.Aging (Albany NY). 2020 Oct 21;12(20):20728-20742. doi: 10.18632/aging.104005. Epub 2020 Oct 21. Aging (Albany NY). 2020. PMID: 33087587 Free PMC article.
-
Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells.J Extracell Vesicles. 2022 Jun;11(6):e12235. doi: 10.1002/jev2.12235. J Extracell Vesicles. 2022. PMID: 35716062 Free PMC article.
-
MicroRNAs in Alzheimer's Disease: Diagnostic Markers or Therapeutic Agents?Front Pharmacol. 2019 Jun 18;10:665. doi: 10.3389/fphar.2019.00665. eCollection 2019. Front Pharmacol. 2019. PMID: 31275145 Free PMC article. Review.
-
Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations.Acta Neuropathol. 2018 Apr;135(4):529-550. doi: 10.1007/s00401-017-1803-x. Epub 2018 Jan 4. Acta Neuropathol. 2018. PMID: 29302779 Free PMC article.
-
Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood-Brain Barrier by Using Different Transport Mechanisms.Int J Mol Sci. 2021 Sep 19;22(18):10118. doi: 10.3390/ijms221810118. Int J Mol Sci. 2021. PMID: 34576281 Free PMC article. Review.
References
-
- Selkoe DJ. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neuro. 2007;8:449–509. - PubMed
-
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1811:639–649. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

