Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine
- PMID: 27523740
- PMCID: PMC7126718
- DOI: 10.1016/j.vaccine.2016.07.051
Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine
Abstract
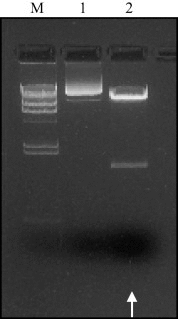
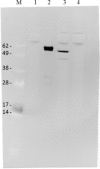
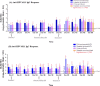
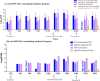
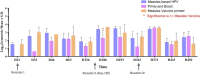
Human papillomavirus (HPV) infection is the most common sexually transmitted disease worldwide. HPVs are oncogenic small double-stranded DNA viruses that are the primary causal agent of cervical cancer and other types of cancers, including in the anus, oropharynx, vagina, vulva, and penis. Prophylactic vaccination against HPV is an attractive strategy for preventing cervical cancer and some other types of cancers. However, there are few safe and effective vaccines against HPV infections. Current first-generation commercial HPV vaccines are expensive to produce and deliver. The goal of this study was to develop an alternate potent HPV recombinant L1-based vaccines by producing HPV virus-like particles into a vaccine that is currently used worldwide. Live attenuated measles virus (MV) vaccines have a well-established safety and efficacy record, and recombinant MV (rMV) produced by reverse genetics may be useful for generating candidate HPV vaccines to meet the needs of the developing world. We studied in non-human primate rMV-vectored HPV vaccine in parallel with a classical alum adjuvant recombinant HPV16L1 and 18L1 protein vaccine produced in Pichia pastoris. A combined prime-boost approach using both vaccines was evaluated, as well as immune interference due to pre-existing immunity against the MV. The humoral immune response induced by the MV, Pichia-expressed vaccine, and their combination as priming and boosting approaches was found to elicit HPV16L1 and 18L1 specific total IgG and neutralizing antibody titres. Pre-existing antibodies against measles did not prevent the immune response against HPV16L1 and 18L1.
Keywords: HPV16L1; HPV18L1; Pichia expressed; Prophylactic vaccine; Recombinant measles.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Physicochemical characterization and immunological properties of Pichia pastoris based HPV16L1 and 18L1 virus like particles.Biologicals. 2017 Mar;46:11-22. doi: 10.1016/j.biologicals.2016.12.002. Epub 2016 Dec 21. Biologicals. 2017. PMID: 28012703
-
Whole recombinant Pichia pastoris expressing HPV16 L1 antigen is superior in inducing protection against tumor growth as compared to killed transgenic Leishmania.Hum Vaccin Immunother. 2014;10(12):3499-508. doi: 10.4161/21645515.2014.979606. Hum Vaccin Immunother. 2014. PMID: 25668661 Free PMC article.
-
Effective Neutralizing Antibody Produced in Mice Directly Immunized with Integrated Pichia pastoris Expressing HPV16L1 Protein.Viral Immunol. 2019 Sep;32(7):308-317. doi: 10.1089/vim.2019.0055. Epub 2019 Aug 5. Viral Immunol. 2019. PMID: 31381497
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Worldwide genetic variations in high-risk human papillomaviruses capsid L1 gene and their impact on vaccine efficiency.Gene. 2021 May 25;782:145533. doi: 10.1016/j.gene.2021.145533. Epub 2021 Feb 23. Gene. 2021. PMID: 33636291 Review.
Cited by
-
The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies.Front Immunol. 2020 Jul 21;11:1817. doi: 10.3389/fimmu.2020.01817. eCollection 2020. Front Immunol. 2020. PMID: 32793245 Free PMC article. Review.
-
Versatility of live-attenuated measles viruses as platform technology for recombinant vaccines.NPJ Vaccines. 2022 Oct 15;7(1):119. doi: 10.1038/s41541-022-00543-4. NPJ Vaccines. 2022. PMID: 36243743 Free PMC article. Review.
-
Self-replicating vehicles based on negative strand RNA viruses.Cancer Gene Ther. 2023 Jun;30(6):771-784. doi: 10.1038/s41417-022-00436-7. Epub 2022 Feb 15. Cancer Gene Ther. 2023. PMID: 35169298 Free PMC article. Review.
-
Engineering and combining oncolytic measles virus for cancer therapy.Cytokine Growth Factor Rev. 2020 Dec;56:39-48. doi: 10.1016/j.cytogfr.2020.07.005. Epub 2020 Jul 3. Cytokine Growth Factor Rev. 2020. PMID: 32718830 Free PMC article. Review.
-
Advances in Designing and Developing Vaccines, Drugs and Therapeutic Approaches to Counter Human Papilloma Virus.Front Immunol. 2018 Nov 12;9:2478. doi: 10.3389/fimmu.2018.02478. eCollection 2018. Front Immunol. 2018. PMID: 30483247 Free PMC article. Review.
References
-
- De Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. - PubMed
-
- Santos-López G., Márquez-Domínguez L., Reyes-Leyva J., Vallejo-Ruiz V. General aspects of structure, classification and replication of human papillomavirus. Rev Med Inst Mex Seguro. 2015;53(2):166–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

