Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model
- PMID: 27520877
- PMCID: PMC5167655
- DOI: 10.1016/j.jhep.2016.07.044
Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model
Abstract
Background & aims: We have established a clinically relevant animal model of hepatocellular cancer (HCC) in immune competent mice to elucidate the complex dialog between host immunity and tumors during HCC initiation and progression. Mechanistic findings have been leveraged to develop a clinically feasible anti-tumor chemoimmunotherapeutic strategy.
Methods: Intraperitoneal injection of carbon tetrachloride and intrasplenic inoculation of oncogenic hepatocytes were combined to induce progressive HCCs in fibrotic livers of immunocompetent mice. Immunization and adoptive cell transfer (ACT) were used to dissect the tumor antigen-specific immune response. The ability of the tyrosine kinase inhibitor sunitinib to enhance immunotherapy in the setting of HCC was evaluated.
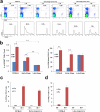
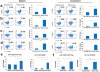
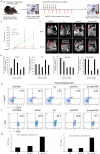
Results: This new mouse model mimics human HCC and reflects its typical features. Tumor-antigen-specific CD8+ T cells maintained a naïve phenotype and remained responsive during early-stage tumor progression. Late tumor progression produced circulating tumor cells, tumor migration into draining lymph nodes, and profound exhaustion of tumor-antigen-specific CD8+ T cells associated with accumulation of programmed cell death protein 1 (PD-1)hi CD8+ T cells and regulatory T cells (Tregs). Sunitinib-mediated tumoricidal effect and Treg suppression synergized with antibody-mediated blockade of PD-1 to powerfully suppress tumor growth and activate anti-tumor immunity.
Conclusion: Treg accumulation and upregulation of PD-1 provide two independent mechanisms to induce profound immune tolerance in HCC. Chemoimmunotherapy using Food and Drug Administration-approved sunitinib with anti-PD-1 antibodies achieved significant tumor control, supporting translation of this approach for the treatment of HCC patients.
Lay summary: In the current study, we have established a clinically relevant mouse model which mimics human liver cancer. Using this unique model, we studied the response of the immune system to this aggressive cancer. Findings from this trial have led to the development of an innovative and clinically feasible chemoimmunotherapeutic strategy.
Keywords: Cancer; Chemoimmunotherapy; Circulating tumor cell (CTC); Hepatocellular cancer (HCC); Immune checkpoint; Programmed cell death protein 1 (PD-1); Regulatory T cells (Tregs); Sunitinib.
Copyright © 2016 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Regression of established hepatocellular carcinoma is induced by chemoimmunotherapy in an orthotopic murine model.Hepatology. 2012 Jan;55(1):141-52. doi: 10.1002/hep.24652. Hepatology. 2012. PMID: 21898502 Free PMC article.
-
Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma.Cancer Immunol Immunother. 2019 Dec;68(12):2055-2066. doi: 10.1007/s00262-019-02427-4. Epub 2019 Nov 13. Cancer Immunol Immunother. 2019. PMID: 31724091 Free PMC article.
-
Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells.Gastroenterology. 2020 Feb;158(3):664-678.e24. doi: 10.1053/j.gastro.2019.10.025. Epub 2019 Oct 31. Gastroenterology. 2020. PMID: 31678303
-
Specific CD8(+) T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis.World J Gastroenterol. 2016 Jul 28;22(28):6469-83. doi: 10.3748/wjg.v22.i28.6469. World J Gastroenterol. 2016. PMID: 27605882 Free PMC article. Review.
-
Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma.World J Gastroenterol. 2018 May 7;24(17):1839-1858. doi: 10.3748/wjg.v24.i17.1839. World J Gastroenterol. 2018. PMID: 29740200 Free PMC article. Review.
Cited by
-
An Oncogenic Hepatocyte-Induced Orthotopic Mouse Model of Hepatocellular Cancer Arising in the Setting of Hepatic Inflammation and Fibrosis.J Vis Exp. 2019 Sep 12;(151):10.3791/59368. doi: 10.3791/59368. J Vis Exp. 2019. PMID: 31566616 Free PMC article.
-
Non-invasive Bioluminescence Monitoring of Hepatocellular Carcinoma Therapy in an HCR Mouse Model.Front Oncol. 2019 Sep 11;9:864. doi: 10.3389/fonc.2019.00864. eCollection 2019. Front Oncol. 2019. PMID: 31572672 Free PMC article.
-
Infiltrating regulatory T cells promote invasiveness of liver cancer cells via inducing epithelial-mesenchymal transition.Transl Cancer Res. 2019 Oct;8(6):2405-2415. doi: 10.21037/tcr.2019.09.54. Transl Cancer Res. 2019. PMID: 35116993 Free PMC article.
-
Heterogeneous responses in hepatocellular carcinoma: the achilles heel of immune checkpoint inhibitors.Am J Cancer Res. 2020 Apr 1;10(4):1085-1102. eCollection 2020. Am J Cancer Res. 2020. PMID: 32368387 Free PMC article. Review.
-
Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma.Front Immunol. 2023 Feb 10;14:1133308. doi: 10.3389/fimmu.2023.1133308. eCollection 2023. Front Immunol. 2023. PMID: 36845131 Free PMC article. Review.
References
-
- Guerra F, Levi Sandri GB. The problem of the most appropriate curative treatment for hepatocellular carcinoma. When to embolize? When to operate? Journal of hepatology. 2015;63(1):280–281. - PubMed
-
- Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. The Lancet Oncology. 2015;16(7):859–870. - PubMed
-
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

