Regulation, Signaling, and Physiological Functions of G-Proteins
- PMID: 27515397
- PMCID: PMC5023507
- DOI: 10.1016/j.jmb.2016.08.002
Regulation, Signaling, and Physiological Functions of G-Proteins
Abstract
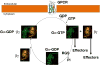
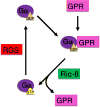
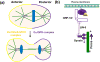
Heterotrimeric guanine-nucleotide-binding regulatory proteins (G-proteins) mainly relay the information from G-protein-coupled receptors (GPCRs) on the plasma membrane to the inside of cells to regulate various biochemical functions. Depending on the targeted cell types, tissues, and organs, these signals modulate diverse physiological functions. The basic schemes of heterotrimeric G-proteins have been outlined. In this review, we briefly summarize what is known about the regulation, signaling, and physiological functions of G-proteins. We then focus on a few less explored areas such as the regulation of G-proteins by non-GPCRs and the physiological functions of G-proteins that cannot be easily explained by the known G-protein signaling pathways. There are new signaling pathways and physiological functions for G-proteins to be discovered and further interrogated. With the advancements in structural and computational biological techniques, we are closer to having a better understanding of how G-proteins are regulated and of the specificity of G-protein interactions with their regulators.
Keywords: G-protein; GPCR; cellular signaling.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



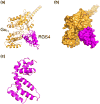
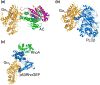
Similar articles
-
Structural mechanism of G protein activation by G protein-coupled receptor.Eur J Pharmacol. 2015 Sep 15;763(Pt B):214-22. doi: 10.1016/j.ejphar.2015.05.016. Epub 2015 May 14. Eur J Pharmacol. 2015. PMID: 25981300 Review.
-
New Insights into Modes of GPCR Activation.Trends Pharmacol Sci. 2018 Apr;39(4):367-386. doi: 10.1016/j.tips.2018.01.001. Epub 2018 Jan 31. Trends Pharmacol Sci. 2018. PMID: 29395118 Review.
-
When a G protein-coupled receptor does not couple to a G protein.Mol Biosyst. 2007 Dec;3(12):849-54. doi: 10.1039/b706343a. Epub 2007 Oct 4. Mol Biosyst. 2007. PMID: 18000562 Review.
-
Fine-tuning of GPCR signals by intracellular G protein modulators.Prog Mol Biol Transl Sci. 2013;115:421-53. doi: 10.1016/B978-0-12-394587-7.00010-5. Prog Mol Biol Transl Sci. 2013. PMID: 23415100 Review.
-
Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling.Semin Cell Dev Biol. 2006 Jun;17(3):383-9. doi: 10.1016/j.semcdb.2006.03.002. Epub 2006 Mar 16. Semin Cell Dev Biol. 2006. PMID: 16647283 Review.
Cited by
-
Insights into Nuclear G-Protein-Coupled Receptors as Therapeutic Targets in Non-Communicable Diseases.Pharmaceuticals (Basel). 2021 May 7;14(5):439. doi: 10.3390/ph14050439. Pharmaceuticals (Basel). 2021. PMID: 34066915 Free PMC article. Review.
-
Signaling mechanisms and physiological functions of G-protein Gα13 in blood vessel formation, bone homeostasis, and cancer.Protein Sci. 2019 Feb;28(2):305-312. doi: 10.1002/pro.3531. Epub 2018 Nov 15. Protein Sci. 2019. PMID: 30345641 Free PMC article. Review.
-
Gα12 and Gα13: Versatility in Physiology and Pathology.Front Cell Dev Biol. 2022 Feb 14;10:809425. doi: 10.3389/fcell.2022.809425. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35237598 Free PMC article. Review.
-
Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms.Cells. 2022 Mar 7;11(5):914. doi: 10.3390/cells11050914. Cells. 2022. PMID: 35269536 Free PMC article. Review.
-
Compartmentalization and regulation of GTP in control of cellular phenotypes.Trends Mol Med. 2022 Sep;28(9):758-769. doi: 10.1016/j.molmed.2022.05.012. Epub 2022 Jun 16. Trends Mol Med. 2022. PMID: 35718686 Free PMC article. Review.
References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–50. - PubMed
-
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. - PubMed
-
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–32. - PubMed
-
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

