Mystixin-7 Peptide Protects Ionotropic Glutamatergic Mechanisms against Glutamate-Induced Excitotoxicity In Vitro
- PMID: 27504123
- PMCID: PMC4967679
- DOI: 10.1155/2016/5151843
Mystixin-7 Peptide Protects Ionotropic Glutamatergic Mechanisms against Glutamate-Induced Excitotoxicity In Vitro
Abstract
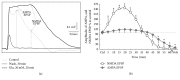
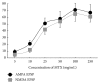
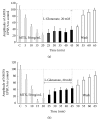
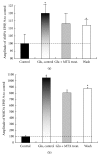
Hyperactivation of the N-methyl-D-aspartic acid type glutamate receptors (NMDARs) causes glutamate excitotoxicity, a process potentially important for many neurological diseases. This study aims to investigate protective effects of the synthetic corticotrophin-releasing factor-like peptide, mystixin-7 (MTX), on model glutamate-induced excitotoxicity in vitro. The technique online monitoring of electrophysiological parameters (excitatory glutamatergic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPAR) and NMDAR-dependent postsynaptic mechanisms) in the olfactory cortex slices was used. Application of L-glutamate in toxic concentration (20 mM) on slices evoked hyperactivation of NMDARs and weaker activation of the AMPARs. Upon further action agonist, the excessive activation of glutamate receptors was replaced by their irreversible blockade. Pretreatment of the slices using MTX in different concentrations (50 and 100 mg/mL) protected both NMDARs and AMPARs from glutamate-induced damage. An enzymatic treatment of MTX reduced hyperactivation of both NMDARs and AMPARs. The present study demonstrated that MTX minipeptide protected the functioning of both NMDARs and AMPARs against glutamate-induced damage. The MTX peptide is a prospective candidate for elaborated medication in treatment of neurological diseases.
Figures
Similar articles
-
Mystixin-7 mini-peptide protects ionotropic glutamatergic mechanisms against oxygen-glucose deprivation in vitro.Neuropeptides. 2016 Apr;56:51-7. doi: 10.1016/j.npep.2015.10.004. Epub 2015 Oct 28. Neuropeptides. 2016. PMID: 26526227
-
Corticotropin-releasing factor-like peptide modifies the AMPA-, NMDA-dependent and GABAB-ergic properties of synaptic transmissions in vitro.Regul Pept. 2014 Jan 10;188:1-4. doi: 10.1016/j.regpep.2013.11.001. Epub 2013 Nov 22. Regul Pept. 2014. PMID: 24275468
-
The Contribution of AMPA and NMDA Receptors to Persistent Firing in the Dorsolateral Prefrontal Cortex in Working Memory.J Neurosci. 2020 Mar 18;40(12):2458-2470. doi: 10.1523/JNEUROSCI.2121-19.2020. Epub 2020 Feb 12. J Neurosci. 2020. PMID: 32051326 Free PMC article.
-
Pre- and postsynaptic ionotropic glutamate receptors in the auditory system of mammals.Hear Res. 2018 May;362:1-13. doi: 10.1016/j.heares.2018.02.007. Epub 2018 Feb 28. Hear Res. 2018. PMID: 29510886 Review.
-
ER to synapse trafficking of NMDA receptors.Front Cell Neurosci. 2014 Nov 27;8:394. doi: 10.3389/fncel.2014.00394. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25505872 Free PMC article. Review.
References
-
- Meldrum B. S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. The Journal of Nutrition. 2000;130(4):1007S–1015S. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

