Rise and fall of vector infectivity during sequential strain displacements by mosquito-borne dengue virus
- PMID: 27500505
- PMCID: PMC5117188
- DOI: 10.1111/jeb.12939
Rise and fall of vector infectivity during sequential strain displacements by mosquito-borne dengue virus
Abstract
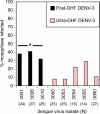
Each of the four serotypes of mosquito-borne dengue virus (DENV-1-4) comprises multiple, genetically distinct strains. Competitive displacement between strains within a serotype is a common feature of DENV epidemiology and can trigger outbreaks of dengue disease. We investigated the mechanisms underlying two sequential displacements by DENV-3 strains in Sri Lanka that each coincided with abrupt increases in dengue haemorrhagic fever (DHF) incidence. First, the post-DHF strain displaced the pre-DHF strain in the 1980s. We have previously shown that post-DHF is more infectious than pre-DHF for the major DENV vector, Aedes aegypti. Then, the ultra-DHF strain evolved in situ from post-DHF and displaced its ancestor in the 2000s. We predicted that ultra-DHF would be more infectious for Ae. aegypti than post-DHF but found that ultra-DHF infected a significantly lower percentage of mosquitoes than post-DHF. We therefore hypothesized that ultra-DHF had effected displacement by disseminating in Ae. aegypti more rapidly than post-DHF, but this was not borne out by a time course of mosquito infection. To elucidate the mechanisms that shape these virus-vector interactions, we tested the impact of RNA interference (RNAi), the principal mosquito defence against DENV, on replication of each of the three DENV strains. Replication of all strains was similar in mosquito cells with dysfunctional RNAi, but in cells with functional RNAi, replication of pre-DHF was significantly suppressed relative to the other two strains. Thus, differences in susceptibility to RNAi may account for the differences in mosquito infectivity between pre-DHF and post-DHF, but other mechanisms underlie the difference between post-DHF and ultra-DHF.
Keywords: Aedes; RNA interference; competitive displacement; dengue virus; mosquito; trade-off; vector.
© 2016 European Society For Evolutionary Biology. Journal of Evolutionary Biology © 2016 European Society For Evolutionary Biology.
Figures
Similar articles
-
Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus.BMC Ecol. 2008 Feb 13;8:1. doi: 10.1186/1472-6785-8-1. BMC Ecol. 2008. PMID: 18269771 Free PMC article.
-
Evolution of dengue in Sri Lanka-changes in the virus, vector, and climate.Int J Infect Dis. 2014 Feb;19:6-12. doi: 10.1016/j.ijid.2013.10.012. Epub 2013 Dec 11. Int J Infect Dis. 2014. PMID: 24334026 Review.
-
Competitive advantage of a dengue 4 virus when co-infecting the mosquito Aedes aegypti with a dengue 1 virus.BMC Infect Dis. 2016 Jul 8;16:318. doi: 10.1186/s12879-016-1666-0. BMC Infect Dis. 2016. PMID: 27390932 Free PMC article.
-
Blood glucose promotes dengue virus infection in the mosquito Aedes aegypti.Parasit Vectors. 2021 Jul 26;14(1):376. doi: 10.1186/s13071-021-04877-1. Parasit Vectors. 2021. PMID: 34311776 Free PMC article.
-
Dengue virus: epidemiology, biology, and disease aetiology.Can J Microbiol. 2021 Oct;67(10):687-702. doi: 10.1139/cjm-2020-0572. Epub 2021 Sep 3. Can J Microbiol. 2021. PMID: 34171205 Review.
Cited by
-
Colonized Sabethes cyaneus, a Sylvatic New World Mosquito Species, Shows a Low Vector Competence for Zika Virus Relative to Aedes aegypti.Viruses. 2018 Aug 16;10(8):434. doi: 10.3390/v10080434. Viruses. 2018. PMID: 30115888 Free PMC article.
-
Emergence of the Asian lineage dengue virus type 3 genotype III in Malaysia.BMC Evol Biol. 2018 Apr 24;18(1):58. doi: 10.1186/s12862-018-1175-4. BMC Evol Biol. 2018. PMID: 29699483 Free PMC article.
-
Current vector research challenges in the greater Mekong subregion for dengue, Malaria, and Other Vector-Borne Diseases: A report from a multisectoral workshop March 2019.PLoS Negl Trop Dis. 2020 Jul 30;14(7):e0008302. doi: 10.1371/journal.pntd.0008302. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32730249 Free PMC article. No abstract available.
-
The Effect of SkitoSnack, an Artificial Blood Meal Replacement, on Aedes aegypti Life History Traits and Gut Microbiota.Sci Rep. 2018 Jul 23;8(1):11023. doi: 10.1038/s41598-018-29415-5. Sci Rep. 2018. PMID: 30038361 Free PMC article.
-
Evolution of resistance to fluoroquinolones by dengue virus serotype 4 provides insight into mechanism of action and consequences for viral fitness.Virology. 2021 Jan 2;552:94-106. doi: 10.1016/j.virol.2020.09.004. Epub 2020 Oct 1. Virology. 2021. PMID: 33120225 Free PMC article.
References
-
- Adams B, Boots M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics. 2010;2:1–10. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

