A radiosensitizing effect of RAD51 inhibition in glioblastoma stem-like cells
- PMID: 27495836
- PMCID: PMC4974671
- DOI: 10.1186/s12885-016-2647-9
A radiosensitizing effect of RAD51 inhibition in glioblastoma stem-like cells
Abstract
Background: Radioresistant glioblastoma stem cells (GSCs) contribute to tumor recurrence and identification of the molecular targets involved in radioresistance mechanisms is likely to enhance therapeutic efficacy. This study analyzed the DNA damage response following ionizing radiation (IR) in 10 GSC lines derived from patients.
Methods: DNA damage was quantified by Comet assay and DNA repair effectors were assessed by Low Density Array. The effect of RAD51 inhibitor, RI-1, was evaluated by comet and annexin V assays.
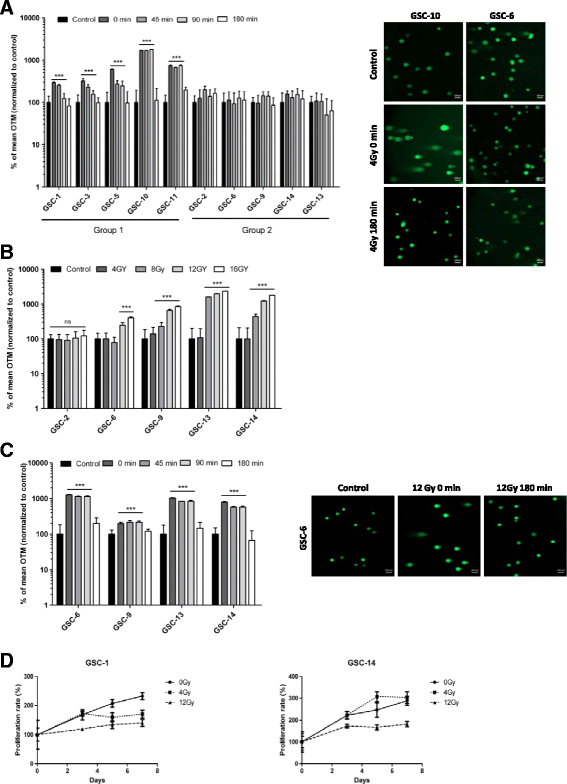
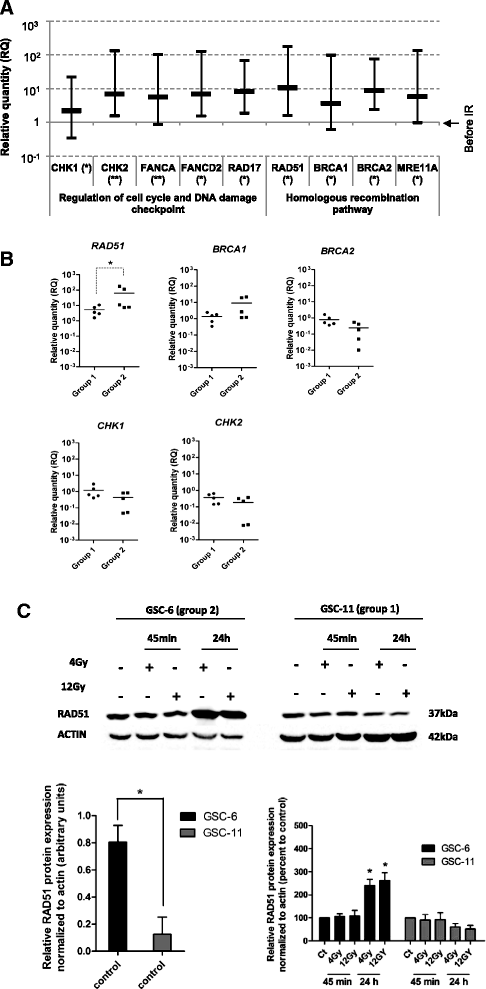
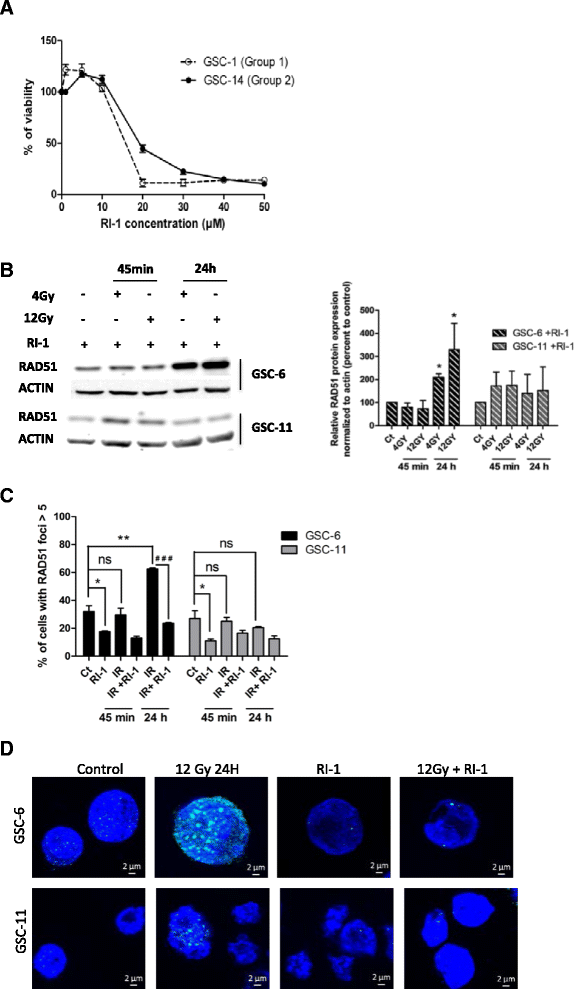
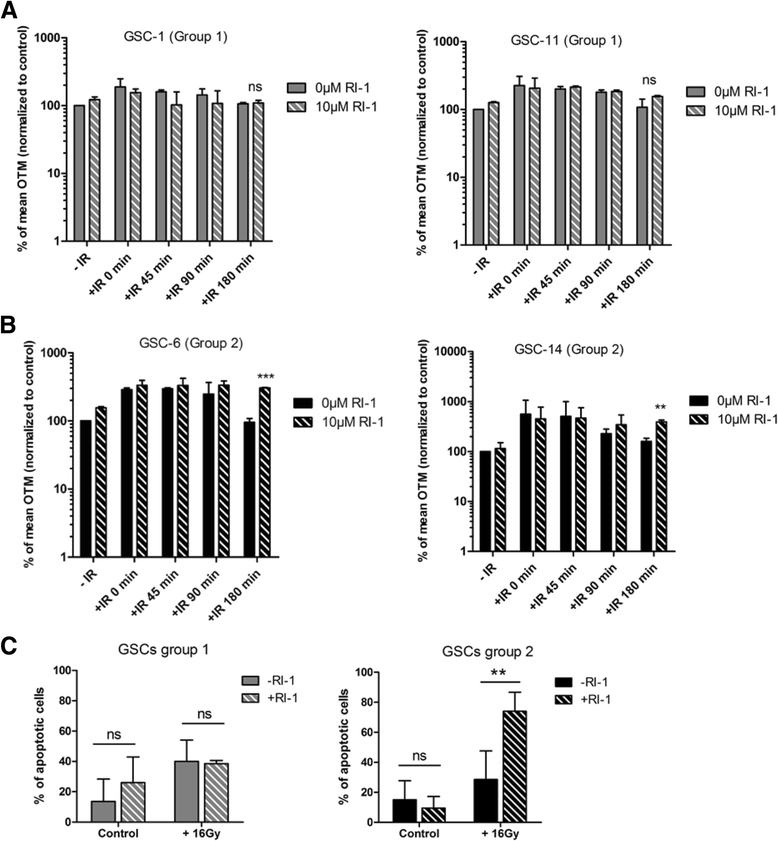
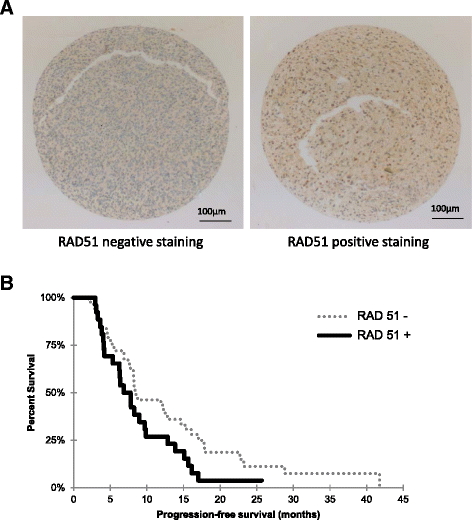
Results: While all GSC lines displayed efficient DNA repair machinery following ionizing radiation, our results demonstrated heterogeneous responses within two distinct groups showing different intrinsic radioresistance, up to 4Gy for group 1 and up to 8Gy for group 2. Radioresistant cell group 2 (comprising 5 out of 10 GSCs) showed significantly higher RAD51 expression after IR. In these cells, inhibition of RAD51 prevented DNA repair up to 180 min after IR and induced apoptosis. In addition, RAD51 protein expression in glioblastoma seems to be associated with poor progression-free survival.
Conclusion: These results underscore the importance of RAD51 in radioresistance of GSCs. RAD51 inhibition could be a therapeutic strategy helping to treat a significant number of glioblastoma, in combination with radiotherapy.
Keywords: Comet assay; Glioblastoma stem cells; RAD51; Radioresistance.
Figures
Similar articles
-
Cell Cycle Changes after Glioblastoma Stem Cell Irradiation: The Major Role of RAD51.Int J Mol Sci. 2018 Oct 3;19(10):3018. doi: 10.3390/ijms19103018. Int J Mol Sci. 2018. PMID: 30282933 Free PMC article.
-
RAD51 Is a Selective DNA Repair Target to Radiosensitize Glioma Stem Cells.Stem Cell Reports. 2017 Jan 10;8(1):125-139. doi: 10.1016/j.stemcr.2016.12.005. Stem Cell Reports. 2017. PMID: 28076755 Free PMC article.
-
Selective Inhibition of Parallel DNA Damage Response Pathways Optimizes Radiosensitization of Glioblastoma Stem-like Cells.Cancer Res. 2015 Oct 15;75(20):4416-28. doi: 10.1158/0008-5472.CAN-14-3790. Epub 2015 Aug 17. Cancer Res. 2015. PMID: 26282173
-
[Cancer stem cells, cornerstone of radioresistance and perspectives for radiosensitization: glioblastoma as an example].Bull Cancer. 2012 Dec;99(12):1153-60. doi: 10.1684/bdc.2012.1666. Bull Cancer. 2012. PMID: 23228708 Review. French.
-
Improving the radiosensitivity of radioresistant and hypoxic glioblastoma.Future Oncol. 2010 Oct;6(10):1591-601. doi: 10.2217/fon.10.123. Future Oncol. 2010. PMID: 21062158 Review.
Cited by
-
Radiotherapeutic Strategies to Overcome Resistance of Breast Cancer Brain Metastases by Considering Immunogenic Aspects of Cancer Stem Cells.Cancers (Basel). 2022 Dec 29;15(1):211. doi: 10.3390/cancers15010211. Cancers (Basel). 2022. PMID: 36612206 Free PMC article. Review.
-
Sinomenine hydrochloride sensitizes cervical cancer cells to ionizing radiation by impairing DNA damage response.Oncol Rep. 2018 Nov;40(5):2886-2895. doi: 10.3892/or.2018.6693. Epub 2018 Sep 10. Oncol Rep. 2018. PMID: 30226618 Free PMC article.
-
RAD51 Inhibitor and Radiation Toxicity in Vestibular Schwannoma.Otolaryngol Head Neck Surg. 2022 Nov;167(5):860-868. doi: 10.1177/01945998221083506. Epub 2022 Mar 1. Otolaryngol Head Neck Surg. 2022. PMID: 35230908 Free PMC article.
-
High expression of RAD51 promotes DNA damage repair and survival in KRAS-mutant lung cancer cells.BMB Rep. 2019 Feb;52(2):151-156. doi: 10.5483/BMBRep.2019.52.2.213. BMB Rep. 2019. PMID: 30638176 Free PMC article.
-
Roles of homologous recombination in response to ionizing radiation-induced DNA damage.Int J Radiat Biol. 2023;99(6):903-914. doi: 10.1080/09553002.2021.1956001. Epub 2021 Aug 4. Int J Radiat Biol. 2023. PMID: 34283012 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

