Hypoxia-Inducible Factor-1α Regulates CD55 in Airway Epithelium
- PMID: 27494303
- PMCID: PMC5248950
- DOI: 10.1165/rcmb.2015-0237OC
Hypoxia-Inducible Factor-1α Regulates CD55 in Airway Epithelium
Abstract
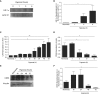
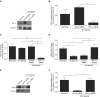
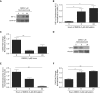
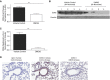
Airway epithelial CD55 down-regulation occurs in several hypoxia-associated pulmonary diseases, but the mechanism is unknown. Using in vivo and in vitro assays of pharmacologic inhibition and gene silencing, the current study investigated the role of hypoxia-inducible factor (HIF)-1α in regulating airway epithelial CD55 expression. Hypoxia down-regulated CD55 expression on small-airway epithelial cells in vitro, and in murine lungs in vivo; the latter was associated with local complement activation. Treatment with pharmacologic inhibition or silencing of HIF-1α during hypoxia-recovered CD55 expression in small-airway epithelial cells. HIF-1α overexpression or blockade, in vitro or in vivo, down-regulated CD55 expression. Collectively, these data show a key role for HIF-1α in regulating the expression of CD55 on airway epithelium.
Keywords: complement regulatory proteins; hypoxia; lung.
Figures


Similar articles
-
Celecoxib Down-Regulates the Hypoxia-Induced Expression of HIF-1α and VEGF Through the PI3K/AKT Pathway in Retinal Pigment Epithelial Cells.Cell Physiol Biochem. 2017;44(4):1640-1650. doi: 10.1159/000485764. Epub 2017 Dec 6. Cell Physiol Biochem. 2017. PMID: 29216640
-
Inverse correlation between metallothioneins and hypoxia-inducible factor 1 alpha in colonocytes and experimental colitis.Biochem Biophys Res Commun. 2011 Dec 16;416(3-4):307-12. doi: 10.1016/j.bbrc.2011.11.031. Epub 2011 Nov 15. Biochem Biophys Res Commun. 2011. PMID: 22108053
-
Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction.Am J Physiol Lung Cell Mol Physiol. 2011 Dec;301(6):L993-L1002. doi: 10.1152/ajplung.00250.2011. Epub 2011 Sep 16. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21926263 Free PMC article.
-
Hypoxia-inducible factor-1α inhibits interleukin-6 and -8 production in gingival epithelial cells during hypoxia.J Periodontal Res. 2017 Feb;52(1):127-134. doi: 10.1111/jre.12377. Epub 2016 Mar 26. J Periodontal Res. 2017. PMID: 27016382
-
Less Is More: Rare Pulmonary Neuroendocrine Cells Function as Critical Sensors in Lung.Dev Cell. 2020 Oct 26;55(2):123-132. doi: 10.1016/j.devcel.2020.09.024. Dev Cell. 2020. PMID: 33108755 Review.
Cited by
-
Our Compliments to the Authors: Decay Accelerating Factor and the Complement System in Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2022 Oct;67(4):415-416. doi: 10.1165/rcmb.2022-0268ED. Am J Respir Cell Mol Biol. 2022. PMID: 35901283 Free PMC article. No abstract available.
-
A Histological Analysis and Detection of Complement Regulatory Protein CD55 in SARS-CoV-2 Infected Lungs.Life (Basel). 2024 Aug 23;14(9):1058. doi: 10.3390/life14091058. Life (Basel). 2024. PMID: 39337843 Free PMC article.
-
Complement Decay-Accelerating Factor is a modulator of influenza A virus lung immunopathology.PLoS Pathog. 2021 Jul 1;17(7):e1009381. doi: 10.1371/journal.ppat.1009381. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34197564 Free PMC article.
-
Regulatory mechanisms of neutrophil migration from the circulation to the airspace.Cell Mol Life Sci. 2021 May;78(9):4095-4124. doi: 10.1007/s00018-021-03768-z. Epub 2021 Feb 5. Cell Mol Life Sci. 2021. PMID: 33544156 Free PMC article. Review.
-
Macrophage HIF-1α Is an Independent Prognostic Indicator in Kidney Cancer.Clin Cancer Res. 2020 Sep 15;26(18):4970-4982. doi: 10.1158/1078-0432.CCR-19-3890. Epub 2020 Jun 25. Clin Cancer Res. 2020. PMID: 32586940 Free PMC article.
References
-
- Wilkes DS. Airway hypoxia, bronchiolar artery revascularization, and obliterative bronchiolitis/bronchiolitis obliterans syndrome: are we there yet? Am J Respir Crit Care Med. 2010;182:136–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

