Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives
- PMID: 27493945
- PMCID: PMC4945779
- DOI: 10.1159/000446052
Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives
Abstract
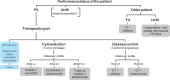
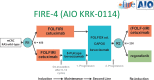
Palliative chemotherapy for metastatic colorectal cancer has undergone substantial changes in recent years. The implementation of modern biologicals in the treatment has substantially improved overall survival up to 30 months. With the increasing number of therapeutic options, the question of optimal treatment sequence arises, which is addressed in current studies like FIRE 4 or STRATEGIC-1. Furthermore, clinical and molecular biomarkers to predict efficacy and tolerability are urgently needed. Today, the detection of activating RAS mutations is the only validated biomarker which precludes patients from anti-EGFR treatment. The detection of BRAF mutation V600E is associated with a very poor prognosis corresponding to a survival of 9-12 months. Prospective trials evaluating an optimal approach to this subgroup are still missing. First results from strategies targeting the aberrant signal transduction are promising and require further validation. Despite the advances so far, life expectancy unfortunately continues to be limited in the majority of patients with metastatic colorectal cancer. New strategies are needed to improve the prognosis. To this end, the identification of Her2/neu as a potential target and first experiences with checkpoint inhibition in patients with mismatch repair-deficient tumors are promising and also require further validation.
Keywords: BRAF mutation; Metastatic colorectal cancer; Palliative chemotherapy; RAS mutation.
Figures
Similar articles
-
Retrospective study of RAS/PIK3CA/BRAF tumor mutations as predictors of response to first-line chemotherapy with bevacizumab in metastatic colorectal cancer patients.BMC Cancer. 2017 Jan 9;17(1):38. doi: 10.1186/s12885-016-2994-6. BMC Cancer. 2017. PMID: 28068936 Free PMC article.
-
Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: the Biomarker Research for anti-EGFR monoclonal Antibodies by Comprehensive Cancer genomics (BREAC) study.Br J Cancer. 2017 Nov 7;117(10):1450-1458. doi: 10.1038/bjc.2017.308. Epub 2017 Oct 3. Br J Cancer. 2017. PMID: 28972961 Free PMC article.
-
The long and winding road to useful predictive factors for anti-EGFR therapy in metastatic colorectal carcinoma: the KRAS/BRAF pathway.Oncology. 2009;77 Suppl 1:57-68. doi: 10.1159/000258497. Epub 2010 Feb 2. Oncology. 2009. PMID: 20130433 Review.
-
Prognostic and Predictive Value of HER2 Amplification in Patients With Metastatic Colorectal Cancer.Clin Colorectal Cancer. 2018 Sep;17(3):198-205. doi: 10.1016/j.clcc.2018.05.006. Epub 2018 May 19. Clin Colorectal Cancer. 2018. PMID: 29866615
-
Promising New Agents for Colorectal Cancer.Curr Treat Options Oncol. 2018 May 11;19(6):29. doi: 10.1007/s11864-018-0543-z. Curr Treat Options Oncol. 2018. PMID: 29752549 Free PMC article. Review.
Cited by
-
Controversies in the treatment of RAS wild-type metastatic colorectal cancer.Clin Transl Oncol. 2021 Apr;23(4):827-839. doi: 10.1007/s12094-020-02475-8. Epub 2020 Aug 13. Clin Transl Oncol. 2021. PMID: 32789773 Free PMC article. Review.
-
hERG1 and HIF-2α Behave as Biomarkers of Positive Response to Bevacizumab in Metastatic Colorectal Cancer Patients.Transl Oncol. 2020 Mar;13(3):100740. doi: 10.1016/j.tranon.2020.01.001. Epub 2020 Feb 25. Transl Oncol. 2020. PMID: 32105990 Free PMC article.
-
ONCOGRAM: study protocol for the evaluation of therapeutic response and survival of metastatic colorectal cancer patients treated according to the guidelines of a chemosensitivity assay, the Oncogramme®.Trials. 2021 Aug 21;22(1):556. doi: 10.1186/s13063-021-05531-y. Trials. 2021. PMID: 34419125 Free PMC article.
-
DNA double-strand breaks repair inhibitors potentiates the combined effect of VP-16 and CDDP in human colorectal adenocarcinoma (LoVo) cells.Mol Biol Rep. 2021 Jan;48(1):709-720. doi: 10.1007/s11033-020-06124-9. Epub 2021 Jan 2. Mol Biol Rep. 2021. PMID: 33389482
-
Novel heavily fucosylated glycans as a promising therapeutic target in colorectal cancer.J Transl Med. 2023 Jul 26;21(1):505. doi: 10.1186/s12967-023-04363-5. J Transl Med. 2023. PMID: 37496011 Free PMC article.
References
-
- Robert Koch-Institut (Hrsg), Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg) Krebs in Deutschland 2011/2012. ed 10. Berlin: 2015.
-
- Bruckner HW, Motwani BT. Chemotherapy of advanced cancer of the colon and rectum. Semin Oncol. 1991;18:443–461. - PubMed
-
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. - PubMed
-
- Modest DP, Hiddemann W, Heinemann V. Chemotherapy of metastatic colorectal cancer (article in German) Internist (Berl) 2014;55:37–42. - PubMed
-
- Modest DP, Stintzing S, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Mohler M, Jung A, Kirchner T, Heinemann V. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33:3718–3726. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous