Pathophysiological Function of ADAMTS Enzymes on Molecular Mechanism of Alzheimer's Disease
- PMID: 27493839
- PMCID: PMC4963191
- DOI: 10.14336/AD.2016.0111
Pathophysiological Function of ADAMTS Enzymes on Molecular Mechanism of Alzheimer's Disease
Abstract
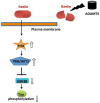
The extracellular matrix (ECM) is an environment that has various enzymes attended in regeneration and restoration processes which is very important to sustain physiological and biological functions of central nervous system (CNS). One of the participating enzyme systems in ECM turnover is matrix metalloproteinases. A disintegrin-like and metalloproteinase with thrombospondin type 1 motifs (ADAMTS) is a unique family of ECM proteases found in mammals. Components of this family may be distinguished from the ADAM (A Disintegrin and Metalloproteinase) family based on the multiple copies of thrombospondin 1-like repeats. The considerable role of the ADAMTS in the CNS continues to develop. Evidences indicate that ADAMTS play an important role in neuroplasticity as well as nervous system pathologies such as Alzheimer's disease (AD). It is hopeful and possible that ADAMTS family members may be utilized to develop therapies for CNS pathologies, ischemic injuries, neurodegenerative and neurological diseases. To understand and provide definitive data on ADAMTS to improve structural and functional recovery in CNS injury and diseases, this review aimed to enlighten the subject extensively to reach certain information on metalloproteinases and related molecules/enzymes. It will be interesting to examine how ADAMTS expression and action would affect the initiation/progression of above-mentioned clinical situations, especially AD.
Keywords: ADAM; ADAMTS; Alzheimer’s disease; matrix metalloproteinases; neurodegeneration.
Figures

Similar articles
-
ADAMTS-4 in central nervous system pathologies.J Neurosci Res. 2017 Sep;95(9):1703-1711. doi: 10.1002/jnr.24021. Epub 2017 Jan 13. J Neurosci Res. 2017. PMID: 28084617 Review.
-
A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease.Biochem Pharmacol. 2019 Jun;164:188-204. doi: 10.1016/j.bcp.2019.03.033. Epub 2019 Mar 21. Biochem Pharmacol. 2019. PMID: 30905657 Free PMC article. Review.
-
ADAMTS: a novel family of extracellular matrix proteases.Int J Biochem Cell Biol. 2001 Jan;33(1):33-44. doi: 10.1016/s1357-2725(00)00061-3. Int J Biochem Cell Biol. 2001. PMID: 11167130 Review.
-
Evolutionary divergence and functions of the ADAM and ADAMTS gene families.Hum Genomics. 2009 Oct;4(1):43-55. doi: 10.1186/1479-7364-4-1-43. Hum Genomics. 2009. PMID: 19951893 Free PMC article.
-
ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the 'usual suspects'.Osteoarthritis Cartilage. 2017 Jul;25(7):1000-1009. doi: 10.1016/j.joca.2017.02.791. Epub 2017 Feb 13. Osteoarthritis Cartilage. 2017. PMID: 28216310 Free PMC article. Review.
Cited by
-
Secretome analysis of oligodendrocytes and precursors reveals their roles as contributors to the extracellular matrix and potential regulators of inflammation.bioRxiv [Preprint]. 2024 Jul 23:2024.07.22.604699. doi: 10.1101/2024.07.22.604699. bioRxiv. 2024. PMID: 39091874 Free PMC article. Preprint.
-
Novel Associations Within the Tumor Microenvironment: Fibulins Meet ADAMTSs.Front Oncol. 2019 Aug 22;9:796. doi: 10.3389/fonc.2019.00796. eCollection 2019. Front Oncol. 2019. PMID: 31508361 Free PMC article. Review.
-
New Insights into ADAMTS Metalloproteases in the Central Nervous System.Biomolecules. 2020 Mar 5;10(3):403. doi: 10.3390/biom10030403. Biomolecules. 2020. PMID: 32150898 Free PMC article. Review.
-
A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients.Clin Exp Immunol. 2018 Apr;192(1):18-32. doi: 10.1111/cei.13087. Epub 2018 Jan 25. Clin Exp Immunol. 2018. PMID: 29194580 Free PMC article.
-
Cell type-specific histone acetylation profiling of Alzheimer's disease subjects and integration with genetics.Front Mol Neurosci. 2023 Jan 6;15:948456. doi: 10.3389/fnmol.2022.948456. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36683855 Free PMC article.
References
-
- Hardy J, Selkoe DJ (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science, 297: 353-356. - PubMed
-
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K (1997). Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem, 272: 556-562. - PubMed
-
- Nardi JB, Martos R, Walden KK, Lampe DJ, Robertson HM (1999). Expression of lacunin, a large multidomain extracellular matrix protein, accompanies morphogenesis of epithelial monolayers in Manduca sexta. Insect Biochem Mol Biol, 29: 883-897. - PubMed
-
- Bork P, Beckmann G (1993). The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol, 231: 539-545. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
