Durable response of glioblastoma to adjuvant therapy consisting of temozolomide and a weekly dose of AMD3100 (plerixafor), a CXCR4 inhibitor, together with lapatinib, metformin and niacinamide
- PMID: 27489862
- PMCID: PMC4965258
- DOI: 10.18632/oncoscience.311
Durable response of glioblastoma to adjuvant therapy consisting of temozolomide and a weekly dose of AMD3100 (plerixafor), a CXCR4 inhibitor, together with lapatinib, metformin and niacinamide
Abstract
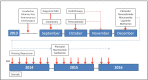
Glioblastoma multiforme (GBM) is a CNS (central nervous system) malignancy with a low cure rate. Median time to progression after standard treatment is 7 months and median overall survival is 15 months [1]. Post-treatment vasculogenesis promoted by recruitment of bone marrow derived cells (BMDCs, CD11b+ myelomonocytes) is one of main mechanisms of GBM resistance to initial chemoradiotherapy treatment [2]. Local secretion of SDF-1, cognate ligand of BMDCs CXCR4 receptors attracts BMDCs to the post-radiation tumor site.[3]. This SDF-1 hypoxia-dependent effect can be blocked by AMD3100 (plerixafor) [4]. We report a GBM case treated after chemo- radiotherapy with plerixafor and a combination of an mTOR, a Sirt1 and an EGFRvIII inhibitor. After one year temozolomide and the EGFRvIII inhibitor were stopped. Plerixafor, and the MTOR and Sirt-1 inhibitors were continued. He is in clinical and radiologic remission 30 months from the initiation of his adjuvant treatment. To our knowledge, this is the first report of a patient treated for over two years with a CXCR4 inhibitor (plerixafor), as part of his adjuvant treatment. We believe there is sufficient experimental evidence to consider AMD3100 (plerixafor) part of the adjuvant treatment of GBM.
Significance: The adjuvant inhibition of GBM vasculogenesis(a process different from local angiogenesis) by specifically blocking the migration of BMDCs to the primary tumor site with inhibitors of the CXCR4/SDF-1 axis represents a potential novel therapeutic approach to GBM. There is significant pre-clinical evidence and validation for its use as demonstrated in a patient derived tumor xenograft model of GBM. Together with other specific anti-tumoral therapies, the active inhibition of vasculogenesis in the adjuvant treatment of GBM is deserving of further exploration.
Keywords: biomedical analytics; glioblastoma; morphoproteomics; plerixafor; preventative and targeted therapy.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice.J Clin Invest. 2010 Mar;120(3):694-705. doi: 10.1172/JCI40283. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179352 Free PMC article.
-
Macrophage Exclusion after Radiation Therapy (MERT): A First in Human Phase I/II Trial using a CXCR4 Inhibitor in Glioblastoma.Clin Cancer Res. 2019 Dec 1;25(23):6948-6957. doi: 10.1158/1078-0432.CCR-19-1421. Epub 2019 Sep 19. Clin Cancer Res. 2019. PMID: 31537527 Free PMC article. Clinical Trial.
-
Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model.J Exp Clin Cancer Res. 2016 Mar 25;35:55. doi: 10.1186/s13046-016-0326-y. J Exp Clin Cancer Res. 2016. PMID: 27015814 Free PMC article.
-
Recruitment of bone marrow derived cells during anti-angiogenic therapy in GBM: the potential of combination strategies.Crit Rev Oncol Hematol. 2014 Oct;92(1):38-48. doi: 10.1016/j.critrevonc.2014.05.001. Epub 2014 May 10. Crit Rev Oncol Hematol. 2014. PMID: 24933160 Review.
-
The Role of a Single Angiogenesis Inhibitor in the Treatment of Recurrent Glioblastoma Multiforme: A Meta-Analysis and Systematic Review.PLoS One. 2016 Mar 23;11(3):e0152170. doi: 10.1371/journal.pone.0152170. eCollection 2016. PLoS One. 2016. PMID: 27007828 Free PMC article. Review.
Cited by
-
The Anti-Angiogenic Effects of Anti-Human Immunodeficiency Virus Drugs.Front Oncol. 2020 May 21;10:806. doi: 10.3389/fonc.2020.00806. eCollection 2020. Front Oncol. 2020. PMID: 32528888 Free PMC article. Review.
-
Repurposing metformin for the treatment of gastrointestinal cancer.World J Gastroenterol. 2021 May 7;27(17):1883-1904. doi: 10.3748/wjg.v27.i17.1883. World J Gastroenterol. 2021. PMID: 34007128 Free PMC article. Review.
-
When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma.Int J Mol Sci. 2018 Feb 1;19(2):436. doi: 10.3390/ijms19020436. Int J Mol Sci. 2018. PMID: 29389898 Free PMC article. Review.
-
Morphoproteomic-Guided Host-Directed Therapy for Tuberculosis.Front Immunol. 2017 Feb 2;8:78. doi: 10.3389/fimmu.2017.00078. eCollection 2017. Front Immunol. 2017. PMID: 28210262 Free PMC article.
-
MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen.Cancers (Basel). 2022 May 23;14(10):2563. doi: 10.3390/cancers14102563. Cancers (Basel). 2022. PMID: 35626167 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. Radiotherapy plus concomitant and adjuvant tomozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–986. - PubMed
-
- Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J. Biol. Chem. 2001;276(17):14153–14160. - PubMed
-
- Brown RE. Morphogenomics and morphoporteomics: a role for anatomic pathology in personalized medicine. Arch Pathol. Lab. Med. 2009;133(4):568–579. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

