A rational two-step approach to KRAS mutation testing in colorectal cancer using high resolution melting analysis and pyrosequencing
- PMID: 27485514
- PMCID: PMC4971616
- DOI: 10.1186/s12885-016-2589-2
A rational two-step approach to KRAS mutation testing in colorectal cancer using high resolution melting analysis and pyrosequencing
Abstract
Background: KRAS mutation testing is mandatory in the management of metastatic colorectal cancer prior to treatment with anti-EGFR antibodies as patients whose tumors express mutant KRAS do not benefit from these agents. Although the U.S. Food and Drug Administration has recently approved two in-vitro diagnostics kits for determination of KRAS status, there is generally no consensus on the preferred method and new tests are continuously being developed. Most of these techniques focus on the hotspot mutations at codons 12 and 13 of the KRAS gene.
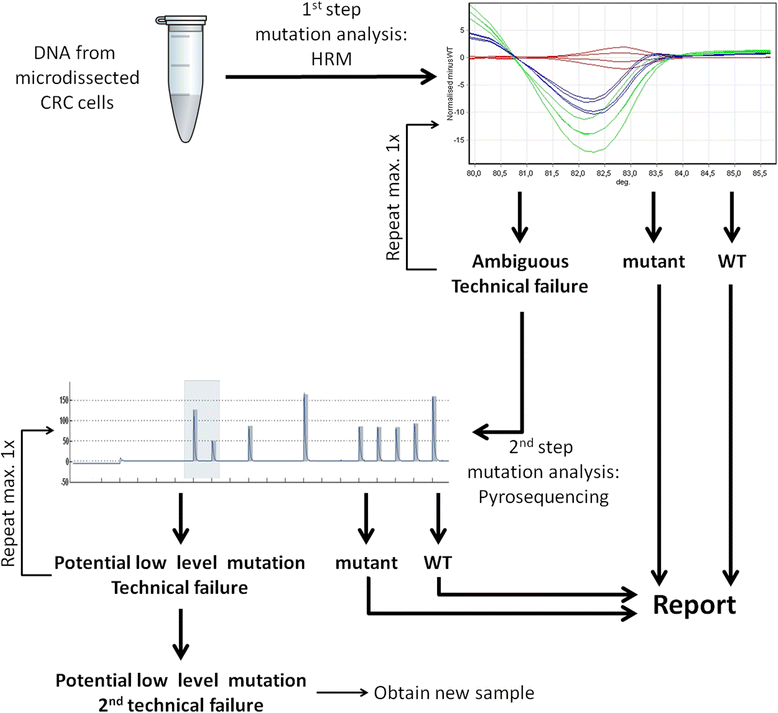
Methods: We describe a two-step approach to KRAS codon 12/13 mutation testing involving high resolution melting analysis (HRM) followed by pyrosequencing using the Therascreen KRAS Pyro kit (Qiagen) of only those samples that are not clearly identified as KRAS wildtype or mutant by HRM. First, we determined KRAS status in a panel of 61 colorectal cancer samples using both methods to compare technical performance and concordance of results. Subsequently, we evaluated practicability and costs of our concept in an independent set of 120 colorectal cancer samples in a routine diagnostic setting.
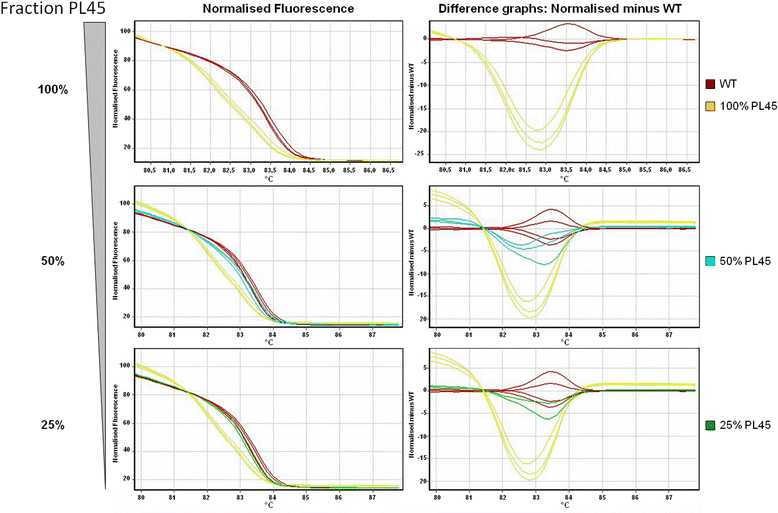
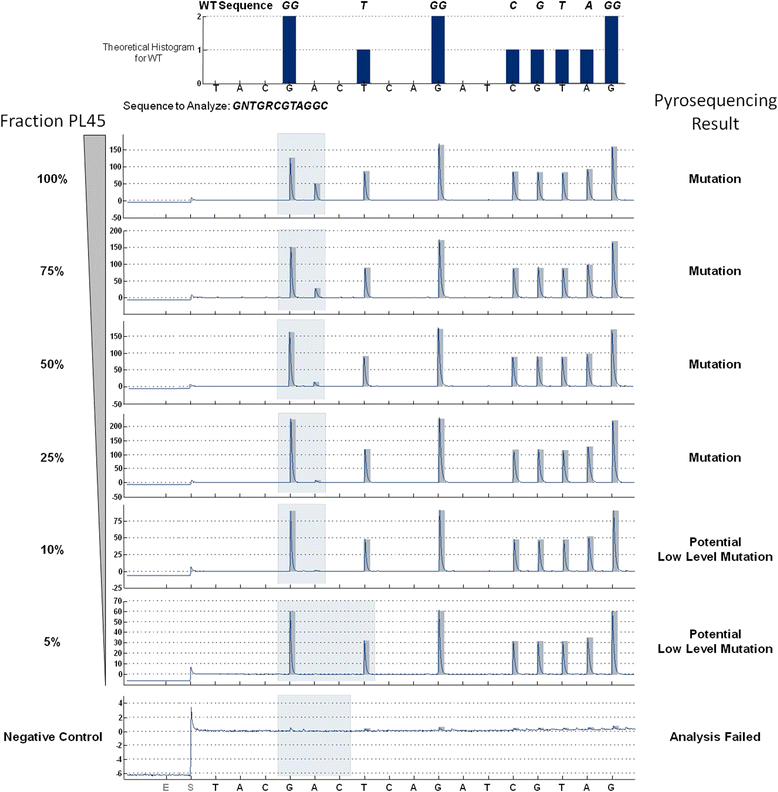
Results: HRM and pyrosequencing appeared to be equally sensitive, allowing for clear detection of mutant alleles at a mutant allele frequency ≥12.5 %. Pyrosequencing yielded more exploitable results due to lower input requirements and a lower rate of analysis failures. KRAS codon 12/13 status was called concordantly for 98.2 % (56/57) of all samples that could be successfully analysed by both methods and 100 % (19/19) of samples that were identified mutant by HRM. Reviewing the actual effort and expenses for KRAS mutation testing in our laboratory revealed, that the selective use of pyrosequencing for only those samples that could not be analysed by HRM increased the fraction of valid results from 87.5 % for HRM alone to 99.2 % (119/120) while allowing for a net reduction of operational costs of >75 % compared to pyrosequencing alone.
Conclusions: Combination of HRM and pyrosequencing in a two-step diagnostic procedure constitutes a reliable and economic analysis platform for KRAS mutation testing in colorectal cancer in a clinical setting.
Keywords: Colorectal cancer; High resolution melting analysis; KRAS mutation; Pyrosequencing.
Figures
Similar articles
-
KRAS gene mutation in a series of unselected colorectal carcinoma patients with prognostic morphological correlations: a pyrosequencing method improved by nested PCR.Exp Mol Pathol. 2015 Jun;98(3):563-7. doi: 10.1016/j.yexmp.2015.03.038. Epub 2015 Mar 31. Exp Mol Pathol. 2015. PMID: 25835782
-
KRAS and BRAF mutation analysis in routine molecular diagnostics: comparison of three testing methods on formalin-fixed, paraffin-embedded tumor-derived DNA.J Mol Diagn. 2012 May-Jun;14(3):247-55. doi: 10.1016/j.jmoldx.2012.01.011. Epub 2012 Mar 14. J Mol Diagn. 2012. PMID: 22425762
-
Comparative analysis of pyrosequencing and QMC-PCR in conjunction with high resolution melting for KRAS/BRAF mutation detection.Int J Exp Pathol. 2010 Dec;91(6):500-5. doi: 10.1111/j.1365-2613.2010.00733.x. Epub 2010 Sep 7. Int J Exp Pathol. 2010. PMID: 21199003 Free PMC article.
-
KRAS mutation testing in colorectal cancer.Adv Anat Pathol. 2009 Jul;16(4):196-203. doi: 10.1097/PAP.0b013e3181a9d4ed. Adv Anat Pathol. 2009. PMID: 19546608 Review.
-
Molecular epidemiology and diagnostics of KRAS mutations in human cancer.Cancer Metastasis Rev. 2020 Dec;39(4):1029-1038. doi: 10.1007/s10555-020-09915-5. Cancer Metastasis Rev. 2020. PMID: 32725342 Free PMC article. Review.
Cited by
-
Performance of probe polymerization-conjunction-agarose gel electrophoresis in the rapid detection of KRAS gene mutation.Genet Mol Biol. 2018 Jul/Sept.;41(3):555-561. doi: 10.1590/1678-4685-GMB-2017-0197. Epub 2018 Jul 16. Genet Mol Biol. 2018. PMID: 30080912 Free PMC article.
-
Progression inference for somatic mutations in cancer.Heliyon. 2017 Apr 11;3(4):e00277. doi: 10.1016/j.heliyon.2017.e00277. eCollection 2017 Apr. Heliyon. 2017. PMID: 28492066 Free PMC article.
References
-
- De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–1820. doi: 10.1001/jama.2010.1535. - DOI - PubMed
-
- De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19(3):508–515. doi: 10.1093/annonc/mdm496. - DOI - PubMed
-
- Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboue R, Tuech JJ, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166–1169. doi: 10.1038/sj.bjc.6603685. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

