A FRET-based method for monitoring septin polymerization and binding of septin-associated proteins
- PMID: 27473902
- PMCID: PMC5021511
- DOI: 10.1016/bs.mcb.2016.03.024
A FRET-based method for monitoring septin polymerization and binding of septin-associated proteins
Abstract
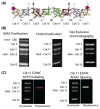
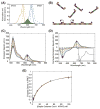
Much about septin function has been inferred from in vivo studies using mainly genetic methods, and much of what we know about septin organization has been obtained through examination of static structures in vitro primarily by electron microscopy. Deeper mechanistic insight requires real-time analysis of the dynamics of the assembly of septin-based structures and how other proteins associate with them. We describe here a Förster resonance energy transfer (FRET)-based approach for measuring in vitro the rate and extent of filament formation from septin complexes, binding of other proteins to septin structures, and the apparent affinities of these interactions. FRET is particularly well suited for interrogating protein-protein interactions, especially on a rapid timescale; the spectral change provides an unambiguous indication of whether two elements within the system under study are associating and serves as a molecular-level "ruler" because it is very sensitive to the separation between the donor and acceptor fluorophores over biologically relevant distances (≤10nm). The necessary procedures involve generation of appropriate cysteine-less and single cysteine-containing septin variants, expression and purification of the heterooctameric complexes containing them, efficient labeling of the purified complexes with desired fluorophores, fluorimetric measurement of FRET, and appropriate safeguards and controls in data acquisition and analysis. Our methods can be used to interrogate the effects of buffer conditions, small molecules, and septin-binding proteins on septin filament assembly or stability; determine the effect of alternative septin subunits, mutational alterations, or posttranslational modifications on assembly; and, delineate the location of septin-binding proteins.
Keywords: Dye labeling; Filament formation; Fluorescence; Förster resonance energy transfer; Protein complexes; Protein engineering; Protein purification; Protein–protein interaction; Site-directed mutagenesis; Supramolecular organization.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
A Förster Resonance Energy Transfer (FRET)-based System Provides Insight into the Ordered Assembly of Yeast Septin Hetero-octamers.J Biol Chem. 2015 Nov 20;290(47):28388-28401. doi: 10.1074/jbc.M115.683128. Epub 2015 Sep 28. J Biol Chem. 2015. PMID: 26416886 Free PMC article.
-
Assays for genetic dissection of septin filament assembly in yeast, from de novo folding through polymerization.Methods Cell Biol. 2016;136:99-116. doi: 10.1016/bs.mcb.2016.03.012. Epub 2016 Apr 13. Methods Cell Biol. 2016. PMID: 27473905
-
Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae.Mol Biol Cell. 2012 Feb;23(3):423-32. doi: 10.1091/mbc.E11-10-0850. Epub 2011 Dec 7. Mol Biol Cell. 2012. PMID: 22160597 Free PMC article.
-
Septin structure and function in yeast and beyond.Trends Cell Biol. 2011 Mar;21(3):141-8. doi: 10.1016/j.tcb.2010.11.006. Epub 2010 Dec 20. Trends Cell Biol. 2011. PMID: 21177106 Free PMC article. Review.
-
Advances in quantitative FRET-based methods for studying nucleic acids.Chembiochem. 2012 Sep 24;13(14):1990-2001. doi: 10.1002/cbic.201200400. Epub 2012 Aug 31. Chembiochem. 2012. PMID: 22936620 Review.
Cited by
-
The hierarchical assembly of septins revealed by high-speed AFM.Nat Commun. 2020 Oct 8;11(1):5062. doi: 10.1038/s41467-020-18778-x. Nat Commun. 2020. PMID: 33033254 Free PMC article.
-
Control of septin filament flexibility and bundling by subunit composition and nucleotide interactions.Mol Biol Cell. 2018 Mar 15;29(6):702-712. doi: 10.1091/mbc.E17-10-0608. Epub 2018 Jan 10. Mol Biol Cell. 2018. PMID: 29321251 Free PMC article.
-
Chemical and Biological Strategies for Profiling Protein-Protein Interactions in Living Cells.Chem Asian J. 2023 Jul 17;18(14):e202300226. doi: 10.1002/asia.202300226. Epub 2023 May 5. Chem Asian J. 2023. PMID: 37089007 Free PMC article. Review.
-
Production and analysis of a mammalian septin hetero-octamer complex.Cytoskeleton (Hoboken). 2020 Nov;77(11):485-499. doi: 10.1002/cm.21643. Epub 2020 Nov 23. Cytoskeleton (Hoboken). 2020. PMID: 33185030 Free PMC article.
-
Effects of Bni5 Binding on Septin Filament Organization.J Mol Biol. 2016 Dec 4;428(24 Pt B):4962-4980. doi: 10.1016/j.jmb.2016.10.024. Epub 2016 Oct 30. J Mol Biol. 2016. PMID: 27806918 Free PMC article.
References
-
- Abdi H, Williams LJ. Principal component analysis. WIREs Computational Statistics. 2010;2:433–459.
-
- Al-Soufi W, Novo M, Mosquera M, Rodríguez-Prieto F. Principal component global analysis of series of fluorescence spectra. Reviews in Fluorescence. 2011;2009:23–46.
-
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, … Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8274–8279. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

