PML nuclear body disruption impairs DNA double-strand break sensing and repair in APL
- PMID: 27468685
- PMCID: PMC4973339
- DOI: 10.1038/cddis.2016.115
PML nuclear body disruption impairs DNA double-strand break sensing and repair in APL
Abstract
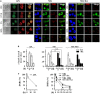
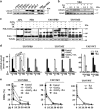
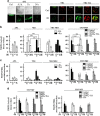
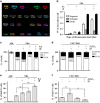
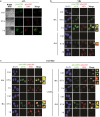
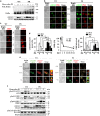
Proteins involved in DNA double-strand break (DSB) repair localize within the promyelocytic leukemia nuclear bodies (PML-NBs), whose disruption is at the root of the acute promyelocytic leukemia (APL) pathogenesis. All-trans-retinoic acid (RA) treatment induces PML-RARα degradation, restores PML-NB functions, and causes terminal cell differentiation of APL blasts. However, the precise role of the APL-associated PML-RARα oncoprotein and PML-NB integrity in the DSB response in APL leukemogenesis and tumor suppression is still lacking. Primary leukemia blasts isolated from APL patients showed high phosphorylation levels of H2AX (γ-H2AX), an initial DSBs sensor. By addressing the consequences of ionizing radiation (IR)-induced DSB response in primary APL blasts and RA-responsive and -resistant myeloid cell lines carrying endogenous or ectopically expressed PML-RARα, before and after treatment with RA, we found that the disruption of PML-NBs is associated with delayed DSB response, as revealed by the impaired kinetic of disappearance of γ-H2AX and 53BP1 foci and activation of ATM and of its substrates H2AX, NBN, and CHK2. The disruption of PML-NB integrity by PML-RARα also affects the IR-induced DSB response in a preleukemic mouse model of APL in vivo. We propose the oncoprotein-dependent PML-NB disruption and DDR impairment as relevant early events in APL tumorigenesis.
Figures


Similar articles
-
Caspases mediate retinoic acid-induced degradation of the acute promyelocytic leukemia PML/RARalpha fusion protein.Blood. 1998 Oct 1;92(7):2244-51. Blood. 1998. PMID: 9746761
-
Promyelocytic leukemia nuclear bodies support a late step in DNA double-strand break repair by homologous recombination.J Cell Biochem. 2012 May;113(5):1787-99. doi: 10.1002/jcb.24050. J Cell Biochem. 2012. PMID: 22213200 Free PMC article.
-
Promyelocytic leukemia activates Chk2 by mediating Chk2 autophosphorylation.J Biol Chem. 2006 Sep 8;281(36):26645-54. doi: 10.1074/jbc.M604391200. Epub 2006 Jul 11. J Biol Chem. 2006. PMID: 16835227
-
Understanding the molecular pathogenesis of acute promyelocytic leukemia.Best Pract Res Clin Haematol. 2014 Mar;27(1):3-9. doi: 10.1016/j.beha.2014.04.006. Epub 2014 Apr 13. Best Pract Res Clin Haematol. 2014. PMID: 24907012 Review.
-
The functional roles of PML nuclear bodies in genome maintenance.Mutat Res. 2018 May;809:99-107. doi: 10.1016/j.mrfmmm.2017.05.002. Epub 2017 May 5. Mutat Res. 2018. PMID: 28521962 Review.
Cited by
-
PML Bodies in Mitosis.Cells. 2019 Aug 14;8(8):893. doi: 10.3390/cells8080893. Cells. 2019. PMID: 31416160 Free PMC article. Review.
-
Promyelocytic Leukemia Proteins Regulate Fanconi Anemia Gene Expression.Int J Mol Sci. 2021 Jul 21;22(15):7782. doi: 10.3390/ijms22157782. Int J Mol Sci. 2021. PMID: 34360546 Free PMC article.
-
Pml nuclear body disruption cooperates in APL pathogenesis and impairs DNA damage repair pathways in mice.Blood. 2018 Feb 8;131(6):636-648. doi: 10.1182/blood-2017-07-794784. Epub 2017 Nov 30. Blood. 2018. PMID: 29191918 Free PMC article.
-
In vivo temporal resolution of acute promyelocytic leukemia progression reveals a role of Klf4 in suppressing early leukemic transformation.Genes Dev. 2022 Apr 1;36(7-8):451-467. doi: 10.1101/gad.349115.121. Epub 2022 Apr 21. Genes Dev. 2022. PMID: 35450883 Free PMC article.
-
More than Meets the ISG15: Emerging Roles in the DNA Damage Response and Beyond.Biomolecules. 2020 Nov 15;10(11):1557. doi: 10.3390/biom10111557. Biomolecules. 2020. PMID: 33203188 Free PMC article. Review.
References
-
- Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat Rev 2005; 31: 603–617. - PubMed
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273: 5858–5868. - PubMed
-
- di Masi A, Viganotti M, Polticelli F, Ascenzi P, Tanzarella C, Antoccia A. The R215W mutation in NBS1 impairs gamma-H2AX binding and affects DNA repair: molecular bases for the severe phenotype of 657del5/R215W Nijmegen breakage syndrome patients. Biochem Biophys Res Commun 2008; 369: 835–840. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

