CD4+ T Cells Recognizing PE/PPE Antigens Directly or via Cross Reactivity Are Protective against Pulmonary Mycobacterium tuberculosis Infection
- PMID: 27467705
- PMCID: PMC4965174
- DOI: 10.1371/journal.ppat.1005770
CD4+ T Cells Recognizing PE/PPE Antigens Directly or via Cross Reactivity Are Protective against Pulmonary Mycobacterium tuberculosis Infection
Abstract
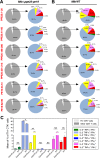
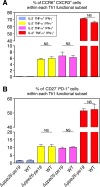
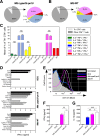
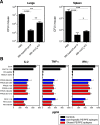
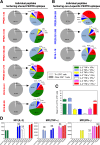
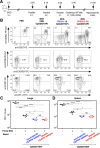
Mycobacterium tuberculosis (Mtb), possesses at least three type VII secretion systems, ESX-1, -3 and -5 that are actively involved in pathogenesis and host-pathogen interaction. We recently showed that an attenuated Mtb vaccine candidate (Mtb Δppe25-pe19), which lacks the characteristic ESX-5-associated pe/ppe genes, but harbors all other components of the ESX-5 system, induces CD4+ T-cell immune responses against non-esx-5-associated PE/PPE protein homologs. These T cells strongly cross-recognize the missing esx-5-associated PE/PPE proteins. Here, we characterized the fine composition of the functional cross-reactive Th1 effector subsets specific to the shared PE/PPE epitopes in mice immunized with the Mtb Δppe25-pe19 vaccine candidate. We provide evidence that the Mtb Δppe25-pe19 strain, despite its significant attenuation, is comparable to the WT Mtb strain with regard to: (i) its antigenic repertoire related to the different ESX systems, (ii) the induced Th1 effector subset composition, (iii) the differentiation status of the Th1 cells induced, and (iv) its particular features at stimulating the innate immune response. Indeed, we found significant contribution of PE/PPE-specific Th1 effector cells in the protective immunity against pulmonary Mtb infection. These results offer detailed insights into the immune mechanisms underlying the remarkable protective efficacy of the live attenuated Mtb Δppe25-pe19 vaccine candidate, as well as the specific potential of PE/PPE proteins as protective immunogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion: encoded PE-PPE proteins predicts vaccine potential.Cell Host Microbe. 2012 Apr 19;11(4):352-63. doi: 10.1016/j.chom.2012.03.003. Cell Host Microbe. 2012. PMID: 22520463
-
Vaccine potential of ESAT-6 protein fused with consensus CD4+ T-cell epitopes of PE/PPE proteins against highly pathogenic Mycobacterium tuberculosis strain HN878.Biochem Biophys Res Commun. 2018 Sep 18;503(4):2195-2201. doi: 10.1016/j.bbrc.2018.06.017. Epub 2018 Aug 5. Biochem Biophys Res Commun. 2018. PMID: 29894686
-
In Vivo Antigen Expression Regulates CD4 T Cell Differentiation and Vaccine Efficacy against Mycobacterium tuberculosis Infection.mBio. 2021 Apr 20;12(2):e00226-21. doi: 10.1128/mBio.00226-21. mBio. 2021. PMID: 33879592 Free PMC article.
-
Expression and regulatory networks of Mycobacterium tuberculosis PE/PPE family antigens.J Cell Physiol. 2019 Jun;234(6):7742-7751. doi: 10.1002/jcp.27608. Epub 2018 Nov 27. J Cell Physiol. 2019. PMID: 30478834 Review.
-
PE and PPE Genes: A Tale of Conservation and Diversity.Adv Exp Med Biol. 2017;1019:191-207. doi: 10.1007/978-3-319-64371-7_10. Adv Exp Med Biol. 2017. PMID: 29116636 Review.
Cited by
-
Mycobacteriaceae Phenome Atlas (MPA): A Standardized Atlas for the Mycobacteriaceae Phenome Based on Heterogeneous Sources.Phenomics. 2023 Jun 13;3(5):439-456. doi: 10.1007/s43657-023-00101-5. eCollection 2023 Oct. Phenomics. 2023. PMID: 37881319 Free PMC article.
-
Mycobacterium tuberculosis PE/PPE proteins enhance the production of reactive oxygen species and formation of neutrophil extracellular traps.Front Immunol. 2023 Aug 22;14:1206529. doi: 10.3389/fimmu.2023.1206529. eCollection 2023. Front Immunol. 2023. PMID: 37675111 Free PMC article.
-
TBVAC2020: Advancing Tuberculosis Vaccines from Discovery to Clinical Development.Front Immunol. 2017 Oct 4;8:1203. doi: 10.3389/fimmu.2017.01203. eCollection 2017. Front Immunol. 2017. PMID: 29046674 Free PMC article. Review.
-
Evolutionary genomic and bacteria GWAS analysis of Mycobacterium avium subsp. paratuberculosis and dairy cattle Johne's disease phenotypes.Appl Environ Microbiol. 2021 Apr 15;87(8):e02570-20. doi: 10.1128/AEM.02570-20. Epub 2021 Feb 5. Appl Environ Microbiol. 2021. PMID: 33547057 Free PMC article.
-
The Macrophage: A Disputed Fortress in the Battle against Mycobacterium tuberculosis.Front Microbiol. 2017 Nov 23;8:2284. doi: 10.3389/fmicb.2017.02284. eCollection 2017. Front Microbiol. 2017. PMID: 29218036 Free PMC article. Review.
References
-
- World Health Organization, Global Tuberculosis Report 2015, Geneva, Switzerland. http://wwwwhoint/tb/publications/global_report/en/. 2015.
-
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nature medicine. 2003;9(5):533–9. - PubMed
-
- Sayes F, Sun L, Di Luca M, Simeone R, Degaiffier N, Fiette L, et al. Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion: encoded PE-PPE proteins predicts vaccine potential. Cell host & microbe. 2012;11(4):352–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

