Heparanase: A Potential New Factor Involved in the Renal Epithelial Mesenchymal Transition (EMT) Induced by Ischemia/Reperfusion (I/R) Injury
- PMID: 27467172
- PMCID: PMC4965068
- DOI: 10.1371/journal.pone.0160074
Heparanase: A Potential New Factor Involved in the Renal Epithelial Mesenchymal Transition (EMT) Induced by Ischemia/Reperfusion (I/R) Injury
Erratum in
-
Correction: Heparanase: A Potential New Factor Involved in the Renal Epithelial Mesenchymal Transition (EMT) Induced by Ischemia/Reperfusion (I/R) Injury.PLoS One. 2017 Apr 10;12(4):e0175618. doi: 10.1371/journal.pone.0175618. eCollection 2017. PLoS One. 2017. PMID: 28394941 Free PMC article.
Abstract
Background: Ischemia/reperfusion (I/R) is an important cause of acute renal failure and delayed graft function, and it may induce chronic renal damage by activating epithelial to mesenchymal transition (EMT) of renal tubular cells. Heparanase (HPSE), an endoglycosidase that regulates FGF-2 and TGFβ-induced EMT, may have an important role. Therefore, aim of this study was to evaluate its role in the I/R-induced renal pro-fibrotic machinery by employing in vitro and in vivo models.
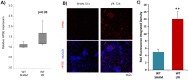
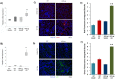
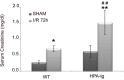
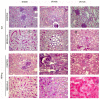
Methods: Wild type (WT) and HPSE-silenced renal tubular cells were subjected to hypoxia and reoxygenation in the presence or absence of SST0001, an inhibitor of HPSE. In vivo, I/R injury was induced by bilateral clamping of renal arteries for 30 min in transgenic mice over-expressing HPSE (HPA-tg) and in their WT littermates. Mice were sacrificed 48 and 72 h after I/R. Gene and protein EMT markers (α-SMA, VIM and FN) were evaluated by bio-molecular and histological methodologies.
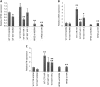
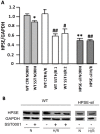
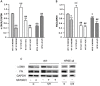
Results: In vitro: hypoxia/reoxygenation (H/R) significantly increased the expression of EMT-markers in WT, but not in HPSE-silenced tubular cells. Notably, EMT was prevented in WT cells by SST0001 treatment. In vivo: I/R induced a remarkable up-regulation of EMT markers in HPA-tg mice after 48-72 h. Noteworthy, these effects were absent in WT animals.
Conclusions: In conclusion, our results add new insights towards understanding the renal biological mechanisms activated by I/R and they demonstrate, for the first time, that HPSE is a pivotal factor involved in the onset and development of I/R-induced EMT. It is plausible that in future the inhibition of this endoglycosidase may represent a new therapeutic approach to minimize/prevent fibrosis and slow down chronic renal disease progression in native and transplanted kidneys.
Conflict of interest statement
Figures

Similar articles
-
Heparanase regulates the M1 polarization of renal macrophages and their crosstalk with renal epithelial tubular cells after ischemia/reperfusion injury.FASEB J. 2018 Feb;32(2):742-756. doi: 10.1096/fj.201700597R. Epub 2018 Jan 4. FASEB J. 2018. PMID: 28970256
-
Everolimus-induced epithelial to mesenchymal transition in immortalized human renal proximal tubular epithelial cells: key role of heparanase.J Transl Med. 2013 Nov 20;11:292. doi: 10.1186/1479-5876-11-292. J Transl Med. 2013. PMID: 24256696 Free PMC article.
-
Heparanase is a key player in renal fibrosis by regulating TGF-β expression and activity.Biochim Biophys Acta. 2014 Sep;1843(9):2122-8. doi: 10.1016/j.bbamcr.2014.06.005. Epub 2014 Jun 15. Biochim Biophys Acta. 2014. PMID: 24937189
-
Impact of heparanase on renal fibrosis.J Transl Med. 2015 Jun 4;13:181. doi: 10.1186/s12967-015-0538-5. J Transl Med. 2015. PMID: 26040666 Free PMC article. Review.
-
Ischemia/reperfusion-associated tubular cells injury in renal transplantation: Can metabolomics inform about mechanisms and help identify new therapeutic targets?Pharmacol Res. 2018 Mar;129:34-43. doi: 10.1016/j.phrs.2017.12.032. Epub 2018 Jan 6. Pharmacol Res. 2018. PMID: 29309901 Review.
Cited by
-
Endothelial Glycocalyx as a Regulator of Fibrotic Processes.Int J Mol Sci. 2021 Mar 15;22(6):2996. doi: 10.3390/ijms22062996. Int J Mol Sci. 2021. PMID: 33804258 Free PMC article. Review.
-
Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19.Biomed J. 2020 Oct;43(5):399-413. doi: 10.1016/j.bj.2020.08.007. Epub 2020 Aug 24. Biomed J. 2020. PMID: 33032965 Free PMC article. Review.
-
Tacrolimus induces fibroblast-to-myofibroblast transition via a TGF-β-dependent mechanism to contribute to renal fibrosis.Am J Physiol Renal Physiol. 2023 May 1;324(5):F433-F445. doi: 10.1152/ajprenal.00226.2022. Epub 2023 Mar 16. Am J Physiol Renal Physiol. 2023. PMID: 36927118 Free PMC article.
-
AKI: an increasingly recognized risk factor for CKD development and progression.J Nephrol. 2020 Dec;33(6):1171-1187. doi: 10.1007/s40620-020-00793-2. Epub 2020 Jul 10. J Nephrol. 2020. PMID: 32651850 Review.
-
Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events.Cells. 2018 Nov 28;7(12):236. doi: 10.3390/cells7120236. Cells. 2018. PMID: 30487472 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

