Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques
- PMID: 27466414
- PMCID: PMC5021426
- DOI: 10.1128/JVI.01163-16
Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques
Abstract
The encouraging results of the RV144 vaccine trial have spurred interest in poxvirus prime-protein boost human immunodeficiency virus (HIV) vaccine modalities as a strategy to induce protective immunity. Because vaccine-induced protective immunity is critically determined by HIV envelope (Env) conformation, significant efforts are directed toward generating soluble trimeric Env immunogens that assume native structures. Using the simian immunodeficiency virus (SIV)-macaque model, we tested the immunogenicity and efficacy of sequential immunizations with DNA (D), modified vaccinia virus Ankara (MVA) (M), and protein immunogens, all expressing virus-like particles (VLPs) displaying membrane-anchored trimeric Env. A single VLP protein boost displaying trimeric gp160 adjuvanted with nanoparticle-encapsulated Toll-like receptor 4/7/8 (TLR4/7/8) agonists, administered 44 weeks after the second MVA immunization, induced up to a 3-fold increase in Env-specific IgG binding titers in serum and mucosa. Importantly, the VLP protein boost increased binding antibody against scaffolded V1V2, antibody-dependent phagocytic activity against VLP-coated beads, and antibody breadth and neutralizing antibody titers against homologous and heterologous tier 1 SIVs. Following 5 weekly intrarectal SIVmac251 challenges, two of seven DNA/MVA and VLP (DM+VLP)-vaccinated animals were completely protected compared to productive infection in all seven DM-vaccinated animals. Vaccinated animals demonstrated stronger acute viral pulldown than controls, but a trend for higher acute viremia was observed in the DM+VLP group, likely due to a slower recall of Gag-specific CD8 T cells. Our findings support immunization with VLPs containing trimeric Env as a strategy to augment protective antibody but underscore the need for optimal engagement of CD8 T cells to achieve robust early viral control.
Importance: The development of an effective HIV vaccine remains a global necessity for preventing HIV infection and reducing the burden of AIDS. While this goal represents a formidable challenge, the modest efficacy of the RV144 trial indicates that multicomponent vaccination regimens that elicit both cellular and humoral immune responses can prevent HIV infection in humans. However, whether protein immunizations synergize with DNA prime-viral vector boosts to enhance cellular and humoral immune responses remains poorly understood. We addressed this question in a nonhuman primate model, and our findings show benefit for sequential protein immunization combined with a potent adjuvant in boosting antibody titers induced by a preceding DNA/MVA immunization. This promising strategy can be further developed to enhance neutralizing antibody responses and boost CD8 T cells to provide robust protection and viral control.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
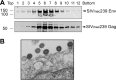




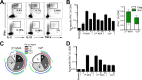


Similar articles
-
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge.J Virol. 2014 Sep 1;88(17):9579-89. doi: 10.1128/JVI.00975-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920805 Free PMC article.
-
Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.J Virol. 2017 Jan 31;91(4):e01844-16. doi: 10.1128/JVI.01844-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928002 Free PMC article.
-
Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge.J Virol. 2018 Jan 2;92(2):e01092-17. doi: 10.1128/JVI.01092-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093095 Free PMC article.
-
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models.Expert Rev Vaccines. 2017 Oct;16(10):973-985. doi: 10.1080/14760584.2017.1371594. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28838267 Free PMC article. Review.
-
HIV vaccine research and discovery in the nonhuman primates model: a unified theory in acquisition prevention and control of SIV infection.Curr Opin HIV AIDS. 2013 Jul;8(4):288-94. doi: 10.1097/COH.0b013e328361cfe8. Curr Opin HIV AIDS. 2013. PMID: 23666390 Free PMC article. Review.
Cited by
-
Impact of Th1 CD4 Follicular Helper T Cell Skewing on Antibody Responses to an HIV-1 Vaccine in Rhesus Macaques.J Virol. 2020 Feb 28;94(6):e01737-19. doi: 10.1128/JVI.01737-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31827000 Free PMC article.
-
Impact of Poxvirus Vector Priming, Protein Coadministration, and Vaccine Intervals on HIV gp120 Vaccine-Elicited Antibody Magnitude and Function in Infant Macaques.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00231-17. doi: 10.1128/CVI.00231-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28814388 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Native-like Env trimers as a platform for HIV-1 vaccine design.Immunol Rev. 2017 Jan;275(1):161-182. doi: 10.1111/imr.12481. Immunol Rev. 2017. PMID: 28133806 Free PMC article. Review.
-
A clade C HIV-1 vaccine protects against heterologous SHIV infection by modulating IgG glycosylation and T helper response in macaques.Sci Immunol. 2022 Jul 22;7(73):eabl4102. doi: 10.1126/sciimmunol.abl4102. Epub 2022 Jul 22. Sci Immunol. 2022. PMID: 35867800 Free PMC article.
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi:10.1056/NEJMoa0908492. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

