Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer
- PMID: 27458171
- PMCID: PMC5392279
- DOI: 10.18632/oncotarget.10775
Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer
Abstract
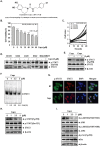
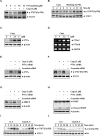
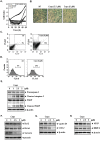
Persistent STAT3 activation is seen in many tumor cells and promotes malignant transformation. Here, we investigated whether capsazepine (Capz), a synthetic analogue of capsaicin, exerts anticancer effects by inhibiting STAT3 activation in prostate cancer cells. Capz inhibited both constitutive and induced STAT3 activation in human prostate carcinoma cells. Capz also inhibited activation of the upstream kinases JAK1/2 and c-Src. The phosphatase inhibitor pervanadate reversed Capz-induced STAT3 inhibition, indicating that the effect of Capz depends on a protein tyrosine phosphatase. Capz treatment increased PTPε protein and mRNA levels. Moreover, siRNA-mediated knockdown of PTPε reversed the Capz-induced induction of PTPε and inhibition of STAT3 activation, indicating that PTPε is crucial for Capz-dependent STAT3 dephosphorylation. Capz also decreased levels of the protein products of various oncogenes, which in turn inhibited proliferation and invasion and induced apoptosis. Finally, intraperitoneal Capz administration decreased tumor growth in a xenograft mouse prostate cancer model and reduced p-STAT3 and Ki-67 expression. These data suggest that Capz is a novel pharmacological inhibitor of STAT3 activation with several anticancer effects in prostate cancer cells.
Keywords: PTPe; STAT3; apoptosis; capsazepine; prostate cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation.Clin Cancer Res. 2007 May 15;13(10):3024-32. doi: 10.1158/1078-0432.CCR-06-2575. Clin Cancer Res. 2007. PMID: 17505005
-
Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells.Food Chem Toxicol. 2011 Jun;49(6):1367-72. doi: 10.1016/j.fct.2011.03.021. Epub 2011 Mar 21. Food Chem Toxicol. 2011. PMID: 21420464
-
Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling.Oncotarget. 2016 Feb 23;7(8):9462-76. doi: 10.18632/oncotarget.7051. Oncotarget. 2016. PMID: 26843613 Free PMC article.
-
Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases.Molecules. 2019 Mar 12;24(5):995. doi: 10.3390/molecules24050995. Molecules. 2019. PMID: 30871017 Free PMC article. Review.
-
The role of STAT3 signaling in mediating tumor resistance to cancer therapy.Curr Drug Targets. 2014;15(14):1341-53. doi: 10.2174/1389450115666141120104146. Curr Drug Targets. 2014. PMID: 25410411 Review.
Cited by
-
Albendazole Exhibits Anti-Neoplastic Actions against Gastric Cancer Cells by Affecting STAT3 and STAT5 Activation by Pleiotropic Mechanism(s).Biomedicines. 2021 Mar 31;9(4):362. doi: 10.3390/biomedicines9040362. Biomedicines. 2021. PMID: 33807326 Free PMC article.
-
Emerging role of exosomes in cancer progression and tumor microenvironment remodeling.J Hematol Oncol. 2022 Jun 28;15(1):83. doi: 10.1186/s13045-022-01305-4. J Hematol Oncol. 2022. PMID: 35765040 Free PMC article. Review.
-
Brassinin alleviates cancer cachexia by suppressing diverse inflammatory mechanisms in mice.MedComm (2020). 2024 May 28;5(6):e558. doi: 10.1002/mco2.558. eCollection 2024 Jun. MedComm (2020). 2024. PMID: 38807976 Free PMC article.
-
Anticancer Activity of Natural and Synthetic Capsaicin Analogs.J Pharmacol Exp Ther. 2018 Mar;364(3):462-473. doi: 10.1124/jpet.117.243691. Epub 2017 Dec 15. J Pharmacol Exp Ther. 2018. PMID: 29246887 Free PMC article. Review.
-
Capsaicin and sorafenib combination treatment exerts synergistic anti‑hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling.Oncol Rep. 2018 Dec;40(6):3235-3248. doi: 10.3892/or.2018.6754. Epub 2018 Oct 1. Oncol Rep. 2018. PMID: 30272354 Free PMC article.
References
-
- Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–154. - PubMed
-
- Masciocchi D, Gelain A, Villa S, Meneghetti F, Barlocco D. Signal transducer and activator of transcription 3 (STAT3): a promising target for anticancer therapy. Future medicinal chemistry. 2011;3:567–597. - PubMed
-
- Chai EZ, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC, Ahn KS, Hui KM, Sethi G. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol Ther. 2015 - PubMed
-
- Kim C, Cho SK, Kapoor S, Kumar A, Vali S, Abbasi T, Kim SH, Sethi G, Ahn KS. beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Molecular carcinogenesis. 2014;53:793–806. - PubMed
-
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

