Hydrophobic-core PEGylated graft copolymer-stabilized nanoparticles composed of insoluble non-nucleoside reverse transcriptase inhibitors exhibit strong anti-HIV activity
- PMID: 27456163
- PMCID: PMC5116409
- DOI: 10.1016/j.nano.2016.07.004
Hydrophobic-core PEGylated graft copolymer-stabilized nanoparticles composed of insoluble non-nucleoside reverse transcriptase inhibitors exhibit strong anti-HIV activity
Abstract
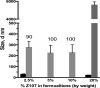
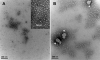
Benzophenone-uracil (BPU) scaffold-derived candidate compounds are efficient non-nucleoside reverse transcriptase inhibitors (NNRTI) with extremely low solubility in water. We proposed to use hydrophobic core (methoxypolyethylene glycol-polylysine) graft copolymer (HC-PGC) technology for stabilizing nanoparticle-based formulations of BPU NNRTI in water. Co-lyophilization of NNRTI/HC-PGC mixtures resulted in dry powders that could be easily reconstituted with the formation of 150-250 nm stable nanoparticles (NP). The NP and HC-PGC were non-toxic in experiments with TZM-bl reporter cells. Nanoparticles containing selected efficient candidate Z107 NNRTI preserved the ability to inhibit HIV-1 reverse transcriptase polymerase activities with no appreciable change of EC50. The formulation with HC-PGC bearing residues of oleic acid resulted in nanoparticles that were nearly identical in anti-HIV-1 potency when compared to Z107 solutions in DMSO (EC50=7.5±3.8 vs. 8.2±5.1 nM). Therefore, hydrophobic core macromolecular stabilizers form nanoparticles with insoluble NNRTI while preserving the antiviral activity of the drug cargo.
Keywords: Benzophenone-uracyl; Copolymer; HIV-1; Microbicide; Nanoparticle; Non-nucleoside reverse transcriptase inhibitors.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
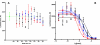
Similar articles
-
Antiretroviral Hydrophobic Core Graft-Copolymer Nanoparticles: The Effectiveness against Mutant HIV-1 Strains and in Vivo Distribution after Topical Application.Pharm Res. 2019 Mar 27;36(5):73. doi: 10.1007/s11095-019-2604-9. Pharm Res. 2019. PMID: 30919089 Free PMC article.
-
Novel concepts in the treatment of human immunodeficiency virus type 1 (HIV-1) infections by HIV-1-specific reverse transcriptase inhibitors.Verh K Acad Geneeskd Belg. 1995;57(6):575-600. Verh K Acad Geneeskd Belg. 1995. PMID: 8686372
-
Characteristics of a group of nonnucleoside reverse transcriptase inhibitors with structural diversity and potent anti-human immunodeficiency virus activity.Leukemia. 1995 Oct;9 Suppl 1:S75-85. Leukemia. 1995. PMID: 7475321
-
Is there a role for non-nucleoside reverse transcriptase inhibitors in the treatment of HIV infection?Infect Agents Dis. 1994 Dec;3(6):313-23. Infect Agents Dis. 1994. PMID: 7534192 Review.
-
Next-generation HIV-1 non-nucleoside reverse transcriptase inhibitors.Curr Opin Investig Drugs. 2006 Feb;7(2):128-35. Curr Opin Investig Drugs. 2006. PMID: 16499282 Review.
Cited by
-
Antiretroviral Hydrophobic Core Graft-Copolymer Nanoparticles: The Effectiveness against Mutant HIV-1 Strains and in Vivo Distribution after Topical Application.Pharm Res. 2019 Mar 27;36(5):73. doi: 10.1007/s11095-019-2604-9. Pharm Res. 2019. PMID: 30919089 Free PMC article.
-
Uracil-Containing Heterodimers of a New Type: Synthesis and Study of Their Anti-Viral Properties.Molecules. 2020 Jul 23;25(15):3350. doi: 10.3390/molecules25153350. Molecules. 2020. PMID: 32717979 Free PMC article.
-
Nanotechnology for virus treatment.Nano Today. 2021 Feb;36:101031. doi: 10.1016/j.nantod.2020.101031. Epub 2020 Dec 1. Nano Today. 2021. PMID: 33519948 Free PMC article. Review.
-
New Derivatives of 5-Substituted Uracils: Potential Agents with a Wide Spectrum of Biological Activity.Molecules. 2022 Apr 30;27(9):2866. doi: 10.3390/molecules27092866. Molecules. 2022. PMID: 35566215 Free PMC article.
-
Silane Modification of Mesoporous Materials for the Optimization of Antiviral Drug Adsorption and Release Capabilities in Vaginal Media.Pharmaceutics. 2021 Sep 7;13(9):1416. doi: 10.3390/pharmaceutics13091416. Pharmaceutics. 2021. PMID: 34575491 Free PMC article.
References
-
- du Toit LC, Pillay V, Choonara YE. Nano-microbicides: Challenges in drug delivery, patient ethics and intellectual property in the war against HIV/AIDS. Adv Drug Deliv Rev. 2010;62:532–46. - PubMed
-
- Sanchez-Rodriguez J, Vacas-Cordoba E, Gomez R, De La Mata FJ, Munoz-Fernandez MA. Nanotech-derived topical microbicides for HIV prevention: The road to clinical development. Antiviral Res. 2015;113:33–48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

