Implantable Device-Related Infection
- PMID: 27454373
- PMCID: PMC5110396
- DOI: 10.1097/SHK.0000000000000692
Implantable Device-Related Infection
Abstract
Over half of the nearly two million healthcare-associated infections can be attributed to indwelling medical devices. In this review, we highlight the difficulty in diagnosing implantable device-related infection and how this leads to a likely underestimate of the prevalence. We then provide a length-scale conceptualization of device-related infection pathogenesis. Within this conceptualization we focus specifically on biofilm formation and the role of host immune and coagulation systems. Using this framework, we describe how current and developing preventative strategies target specific processes along the entire length-scale. In light of the significant time horizon for the development and translation of new preventative technologies, we also emphasize the need for parallel development of in situ treatment strategies. Specific examples of both preventative and treatment strategies and how they align with the length-scale conceptualization are described.
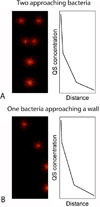
Figures




Similar articles
-
Infectious Diseases Impact on Biomedical Devices and Materials.Biomed Mater Devices. 2022 Oct 28:1-8. doi: 10.1007/s44174-022-00035-y. Online ahead of print. Biomed Mater Devices. 2022. PMID: 38625309 Free PMC article. Review.
-
Bacterial adherence and biofilm formation on medical implants: a review.Proc Inst Mech Eng H. 2014 Oct;228(10):1083-99. doi: 10.1177/0954411914556137. Proc Inst Mech Eng H. 2014. PMID: 25406229 Review.
-
Staphylococcus epidermidis device-related infections: pathogenesis and clinical management.J Pharm Pharmacol. 2008 Dec;60(12):1551-71. doi: 10.1211/jpp/60.12.0001. J Pharm Pharmacol. 2008. PMID: 19000360 Review.
-
Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities.APMIS Suppl. 2014 Dec;(138):1-51. doi: 10.1111/apm.12335. APMIS Suppl. 2014. PMID: 25399808 Review.
-
Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants.J Biomed Mater Res A. 2007 Oct;83(1):10-9. doi: 10.1002/jbm.a.31184. J Biomed Mater Res A. 2007. PMID: 17340599
Cited by
-
Energy-Efficient Integrated Circuit Solutions Toward Miniaturized Closed-Loop Neural Interface Systems.Front Neurosci. 2021 May 31;15:667447. doi: 10.3389/fnins.2021.667447. eCollection 2021. Front Neurosci. 2021. PMID: 34135727 Free PMC article. Review.
-
Staphylococcus aureus Breast Implant Infection Isolates Display Recalcitrance To Antibiotic Pocket Irrigants.Microbiol Spectr. 2023 Feb 14;11(1):e0288422. doi: 10.1128/spectrum.02884-22. Epub 2022 Dec 12. Microbiol Spectr. 2023. PMID: 36507629 Free PMC article.
-
Role of Implantable Drug Delivery Devices with Dual Platform Capabilities in the Prevention and Treatment of Bacterial Osteomyelitis.Bioengineering (Basel). 2022 Feb 6;9(2):65. doi: 10.3390/bioengineering9020065. Bioengineering (Basel). 2022. PMID: 35200418 Free PMC article. Review.
-
Pharmacodynamics of Moxifloxacin, Meropenem, Caspofungin, and Their Combinations against In Vitro Polymicrobial Interkingdom Biofilms.Antimicrob Agents Chemother. 2022 Feb 15;66(2):e0214921. doi: 10.1128/AAC.02149-21. Epub 2021 Dec 20. Antimicrob Agents Chemother. 2022. PMID: 34930026 Free PMC article.
-
Improve Integration of In Vitro Biofilm Body of Knowledge to Support Clinical Breakthroughs in Surgical Site Infection.J Am Acad Orthop Surg Glob Res Rev. 2021 Nov 4;5(11):e20.00217. doi: 10.5435/JAAOSGlobal-D-20-00217. J Am Acad Orthop Surg Glob Res Rev. 2021. PMID: 34748523 Free PMC article. Review.
References
-
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. - PubMed
-
- Weinstein RA, Darouiche RO. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clinical Infectious Diseases. 2001;33(9):1567–1572. - PubMed
-
- Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33(4):414–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

