Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation
- PMID: 27452175
- PMCID: PMC5336327
- DOI: 10.1016/j.stem.2016.06.018
Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation
Abstract
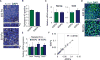
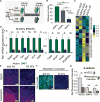
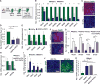
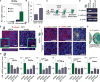
Regenerative medicine is predicated on understanding the mechanisms regulating development and applying these conditions to direct stem cell fate. Embryogenesis is guided by cell-cell and cell-matrix interactions, but it is unclear how these physical cues influence stem cells in culture. We used human embryonic stem cells (hESCs) to examine whether mechanical features of the extracellular microenvironment could differentially modulate mesoderm specification. We found that, on a hydrogel-based compliant matrix, hESCs accumulate β-catenin at cell-cell adhesions and show enhanced Wnt-dependent mesoderm differentiation. Mechanistically, Src-driven ubiquitination of E-cadherin by Cbl-like ubiquitin ligase releases P120-catenin to facilitate transcriptional activity of β-catenin, which initiates and reinforces mesoderm differentiation. By contrast, on a stiff hydrogel matrix, hESCs show elevated integrin-dependent GSK3 and Src activity that promotes β-catenin degradation and inhibits differentiation. Thus, we found that mechanical features of the microenvironmental matrix influence tissue-specific differentiation of hESCs by altering the cellular response to morphogens.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
E-cadherin adhesion-mediated Wnt activation for mesoderm specification in human embryonic stem cells needs a soft mattress.Stem Cell Investig. 2016 Nov 14;3:77. doi: 10.21037/sci.2016.10.12. eCollection 2016. Stem Cell Investig. 2016. PMID: 28066779 Free PMC article. No abstract available.
Similar articles
-
A Regulatory Network Involving β-Catenin, e-Cadherin, PI3k/Akt, and Slug Balances Self-Renewal and Differentiation of Human Pluripotent Stem Cells In Response to Wnt Signaling.Stem Cells. 2015 May;33(5):1419-33. doi: 10.1002/stem.1944. Stem Cells. 2015. PMID: 25538040 Free PMC article.
-
Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells.Dev Cell. 2020 Dec 21;55(6):679-694.e11. doi: 10.1016/j.devcel.2020.10.015. Epub 2020 Nov 17. Dev Cell. 2020. PMID: 33207224 Free PMC article.
-
Wnt/β-catenin-mediated signaling re-activates proliferation of matured cardiomyocytes.Stem Cell Res Ther. 2018 Dec 7;9(1):338. doi: 10.1186/s13287-018-1086-8. Stem Cell Res Ther. 2018. PMID: 30526659 Free PMC article.
-
Extracellular matrix stiffness and Wnt/β-catenin signaling in physiology and disease.Biochem Soc Trans. 2020 Jun 30;48(3):1187-1198. doi: 10.1042/BST20200026. Biochem Soc Trans. 2020. PMID: 32412078 Review.
-
Wnt signaling in development and tissue homeostasis.Development. 2018 Jun 8;145(11):dev146589. doi: 10.1242/dev.146589. Development. 2018. PMID: 29884654 Review.
Cited by
-
Cadherins in early neural development.Cell Mol Life Sci. 2021 May;78(9):4435-4450. doi: 10.1007/s00018-021-03815-9. Epub 2021 Apr 1. Cell Mol Life Sci. 2021. PMID: 33796894 Free PMC article. Review.
-
p120-catenin regulates WNT signaling and EMT in the mouse embryo.Proc Natl Acad Sci U S A. 2019 Aug 20;116(34):16872-16881. doi: 10.1073/pnas.1902843116. Epub 2019 Aug 1. Proc Natl Acad Sci U S A. 2019. PMID: 31371508 Free PMC article.
-
Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells.Nat Mater. 2019 Apr;18(4):397-405. doi: 10.1038/s41563-019-0287-6. Epub 2019 Feb 18. Nat Mater. 2019. PMID: 30778227 Free PMC article.
-
The Use of Pluripotent Stem Cell-Derived Organoids to Study Extracellular Matrix Development during Neural Degeneration.Cells. 2019 Mar 14;8(3):242. doi: 10.3390/cells8030242. Cells. 2019. PMID: 30875781 Free PMC article. Review.
-
Emerging Methods for Enhancing Pluripotent Stem Cell Expansion.Front Cell Dev Biol. 2020 Feb 14;8:70. doi: 10.3389/fcell.2020.00070. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32117992 Free PMC article. Review.
References
-
- Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. - PubMed
-
- Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. - PubMed
-
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. - PubMed
-
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

