DAPK1 Signaling Pathways in Stroke: from Mechanisms to Therapies
- PMID: 27447806
- PMCID: PMC5509806
- DOI: 10.1007/s12035-016-0008-y
DAPK1 Signaling Pathways in Stroke: from Mechanisms to Therapies
Abstract
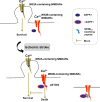
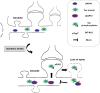
Death-associated protein kinase 1 (DAPK1), a Ca2+/calmodulin (CaM)-dependent serine/threonine protein kinase, plays important roles in diverse apoptosis pathways not only in tumor suppression but also in neuronal cell death. The requirement of DAPK1 catalytic activity for its proposed cell functions and the elevation of catalytic activity of DAPK1 in injured neurons in models of neurological diseases, such as ischemia and epilepsy, validate that DAPK1 can be taken as a potential therapeutic target in these diseases. Recent studies show that DAPK1-NR2B, DAPK1-DANGER, DAPK1-p53, and DAPK1-Tau are currently known pathways in stroke-induced cell death, and blocking these cascades in an acute treatment effectively reduces neuronal loss. In this review, we focus on the role of DAPK1 in neuronal cell death after stroke. We hope to provide exhaustive summaries of relevant studies on DAPK1 signals involved in stroke damage. Therefore, disrupting DAPK1-relevant cell death pathway could be considered as a promising therapeutic approach in stroke.
Keywords: Cell death; DAPK1; Mechanism; Stroke; Therapeutics.
Conflict of interest statement
Competing Interests
The authors declare that they have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81130079 and 91232302 to Y.M. L), the Key Project of United Fund of National Natural and Guangdong Province (U1301223 to H.Y. S), and the Medical Scientific Research Foundation of Guangdong Province, China (A2016304 to S. W).
Figures
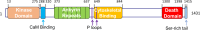

Similar articles
-
Death-Associated Protein Kinase 1 Phosphorylation in Neuronal Cell Death and Neurodegenerative Disease.Int J Mol Sci. 2019 Jun 26;20(13):3131. doi: 10.3390/ijms20133131. Int J Mol Sci. 2019. PMID: 31248062 Free PMC article. Review.
-
A Novel Mechanism of Spine Damages in Stroke via DAPK1 and Tau.Cereb Cortex. 2015 Nov;25(11):4559-71. doi: 10.1093/cercor/bhv096. Epub 2015 May 20. Cereb Cortex. 2015. PMID: 25995053 Free PMC article.
-
DAPK1-p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death.J Neurosci. 2014 May 7;34(19):6546-56. doi: 10.1523/JNEUROSCI.5119-13.2014. J Neurosci. 2014. PMID: 24806680 Free PMC article.
-
Exploring putative inhibitors of Death Associated Protein Kinase 1 (DAPK1) via targeting Gly-Glu-Leu (GEL) and Pro-Glu-Asn (PEN) substrate recognition motifs.J Mol Graph Model. 2017 Oct;77:153-167. doi: 10.1016/j.jmgm.2017.08.001. Epub 2017 Aug 18. J Mol Graph Model. 2017. PMID: 28858643
-
Role of DAPK1 in neuronal cell death, survival and diseases in the nervous system.Int J Dev Neurosci. 2019 May;74:11-17. doi: 10.1016/j.ijdevneu.2019.02.003. Epub 2019 Feb 11. Int J Dev Neurosci. 2019. PMID: 30763607 Review.
Cited by
-
CircRNA 010567 plays a significant role in myocardial infarction via the regulation of the miRNA-141/DAPK1 axis.J Thorac Dis. 2021 Apr;13(4):2447-2459. doi: 10.21037/jtd-21-212. J Thorac Dis. 2021. PMID: 34012592 Free PMC article.
-
Death-associated protein kinase 1 as a therapeutic target for Alzheimer's disease.Transl Neurodegener. 2024 Jan 9;13(1):4. doi: 10.1186/s40035-023-00395-5. Transl Neurodegener. 2024. PMID: 38195518 Free PMC article. Review.
-
Icaritin Alleviates Glutamate-Induced Neuronal Damage by Inactivating GluN2B-Containing NMDARs Through the ERK/DAPK1 Pathway.Front Neurosci. 2021 Feb 22;15:525615. doi: 10.3389/fnins.2021.525615. eCollection 2021. Front Neurosci. 2021. PMID: 33692666 Free PMC article.
-
Pin1-catalyzed conformational regulation after phosphorylation: A distinct checkpoint in cell signaling and drug discovery.Sci Signal. 2024 Jun 18;17(841):eadi8743. doi: 10.1126/scisignal.adi8743. Epub 2024 Jun 18. Sci Signal. 2024. PMID: 38889227 Review.
-
A comprehensive review of stroke-related signaling pathways and treatment in western medicine and traditional Chinese medicine.Front Neurosci. 2023 Jun 7;17:1200061. doi: 10.3389/fnins.2023.1200061. eCollection 2023. Front Neurosci. 2023. PMID: 37351420 Free PMC article. Review.
References
-
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

