Canonical and Non-canonical Reelin Signaling
- PMID: 27445693
- PMCID: PMC4928174
- DOI: 10.3389/fncel.2016.00166
Canonical and Non-canonical Reelin Signaling
Abstract
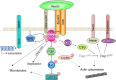
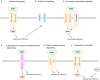
Reelin is a large secreted glycoprotein that is essential for correct neuronal positioning during neurodevelopment and is important for synaptic plasticity in the mature brain. Moreover, Reelin is expressed in many extraneuronal tissues; yet the roles of peripheral Reelin are largely unknown. In the brain, many of Reelin's functions are mediated by a molecular signaling cascade that involves two lipoprotein receptors, apolipoprotein E receptor-2 (Apoer2) and very low density-lipoprotein receptor (Vldlr), the neuronal phosphoprotein Disabled-1 (Dab1), and members of the Src family of protein tyrosine kinases as crucial elements. This core signaling pathway in turn modulates the activity of adaptor proteins and downstream protein kinase cascades, many of which target the neuronal cytoskeleton. However, additional Reelin-binding receptors have been postulated or described, either as coreceptors that are essential for the activation of the "canonical" Reelin signaling cascade involving Apoer2/Vldlr and Dab1, or as receptors that activate alternative or additional signaling pathways. Here we will give an overview of canonical and alternative Reelin signaling pathways, molecular mechanisms involved, and their potential physiological roles in the context of different biological settings.
Keywords: Alzheimer disease; Eph receptor; amyloid precursor protein; coreceptor; integrin; neuronal development; protein kinase; synaptic plasticity.
Figures
Similar articles
-
Receptor clustering is involved in Reelin signaling.Mol Cell Biol. 2004 Feb;24(3):1378-86. doi: 10.1128/MCB.24.3.1378-1386.2004. Mol Cell Biol. 2004. PMID: 14729980 Free PMC article.
-
Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases.Brain Res Mol Brain Res. 2003 Oct 7;117(2):152-9. doi: 10.1016/s0169-328x(03)00295-x. Brain Res Mol Brain Res. 2003. PMID: 14559149
-
Beyond Glycolysis: Aldolase A is a Novel Effector in Reelin Mediated Dendritic Development.bioRxiv [Preprint]. 2024 Jan 12:2024.01.12.575269. doi: 10.1101/2024.01.12.575269. bioRxiv. 2024. Update in: J Neurosci. 2024 Oct 16;44(42):e0072242024. doi: 10.1523/JNEUROSCI.0072-24.2024 PMID: 38260505 Free PMC article. Updated. Preprint.
-
The functions of Reelin in membrane trafficking and cytoskeletal dynamics: implications for neuronal migration, polarization and differentiation.Biochem J. 2017 Sep 7;474(18):3137-3165. doi: 10.1042/BCJ20160628. Biochem J. 2017. PMID: 28887403 Review.
-
New Insights into Reelin-Mediated Signaling Pathways.Front Cell Neurosci. 2016 May 9;10:122. doi: 10.3389/fncel.2016.00122. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242434 Free PMC article. Review.
Cited by
-
Reelin Protects against Colon Pathology via p53 and May Be a Biomarker for Colon Cancer Progression.Biology (Basel). 2022 Sep 26;11(10):1406. doi: 10.3390/biology11101406. Biology (Basel). 2022. PMID: 36290310 Free PMC article.
-
Distal Dendritic Enrichment of HCN1 Channels in Hippocampal CA1 Is Promoted by Estrogen, but Does Not Require Reelin.eNeuro. 2018 Oct 15;5(5):ENEURO.0258-18.2018. doi: 10.1523/ENEURO.0258-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30406178 Free PMC article.
-
Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders.Pharmacol Ther. 2020 Jun;210:107523. doi: 10.1016/j.pharmthera.2020.107523. Epub 2020 Mar 9. Pharmacol Ther. 2020. PMID: 32165138 Free PMC article. Review.
-
C3G Protein, a New Player in Glioblastoma.Int J Mol Sci. 2021 Sep 16;22(18):10018. doi: 10.3390/ijms221810018. Int J Mol Sci. 2021. PMID: 34576182 Free PMC article. Review.
-
APP Protein Family Signaling at the Synapse: Insights from Intracellular APP-Binding Proteins.Front Mol Neurosci. 2017 Mar 30;10:87. doi: 10.3389/fnmol.2017.00087. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28424586 Free PMC article. Review.
References
-
- Andrade N., Komnenovic V., Blake S. M., Jossin Y., Howell B., Goffinet A., et al. . (2007). ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc. Natl. Acad. Sci. U S A 104, 8508–8513. 10.1073/pnas.0611391104 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

