MORC3, a Component of PML Nuclear Bodies, Has a Role in Restricting Herpes Simplex Virus 1 and Human Cytomegalovirus
- PMID: 27440897
- PMCID: PMC5021396
- DOI: 10.1128/JVI.00621-16
MORC3, a Component of PML Nuclear Bodies, Has a Role in Restricting Herpes Simplex Virus 1 and Human Cytomegalovirus
Abstract
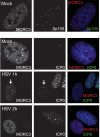
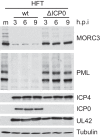
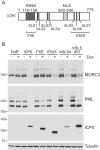
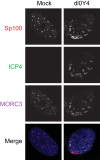
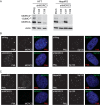
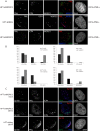
We previously reported that MORC3, a protein associated with promyelocytic leukemia nuclear bodies (PML NBs), is a target of herpes simplex virus 1 (HSV-1) ICP0-mediated degradation (E. Sloan, et al., PLoS Pathog 11:e1005059, 2015, http://dx.doi.org/10.1371/journal.ppat.1005059). Since it is well known that certain other components of the PML NB complex play an important role during an intrinsic immune response to HSV-1 and are also degraded or inactivated by ICP0, here we further investigate the role of MORC3 during HSV-1 infection. We demonstrate that MORC3 has antiviral activity during HSV-1 infection and that this antiviral role is counteracted by ICP0. In addition, MORC3's antiviral role extends to wild-type (wt) human cytomegalovirus (HCMV) infection, as its plaque-forming efficiency increased in MORC3-depleted cells. We found that MORC3 is recruited to sites associated with HSV-1 genomes after their entry into the nucleus of an infected cell, and in wt infections this is followed by its association with ICP0 foci prior to its degradation. The RING finger domain of ICP0 was required for degradation of MORC3, and we confirmed that no other HSV-1 protein is required for the loss of MORC3. We also found that MORC3 is required for fully efficient recruitment of PML, Sp100, hDaxx, and γH2AX to sites associated with HSV-1 genomes entering the host cell nucleus. This study further unravels the intricate ways in which HSV-1 has evolved to counteract the host immune response and reveals a novel function for MORC3 during the host intrinsic immune response.
Importance: Herpesviruses have devised ways to manipulate the host intrinsic immune response to promote their own survival and persistence within the human population. One way in which this is achieved is through degradation or functional inactivation of PML NB proteins, which are recruited to viral genomes in order to repress viral transcription. Because MORC3 associates with PML NBs in uninfected cells and is a target for HSV-1-mediated degradation, we investigated the role of MORC3 during HSV-1 infection. We found that MORC3 is also recruited to viral HSV-1 genomes, and importantly it contributes to the fully efficient recruitment of PML, hDaxx, Sp100, and γH2AX to these sites. Depletion of MORC3 resulted in an increase in ICP0-null HSV-1 and wt HCMV replication and plaque formation; therefore, this study reveals that MORC3 is an antiviral factor which plays an important role during HSV-1 and HCMV infection.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
-
Expression of Human Cytomegalovirus IE1 Leads to Accumulation of Mono-SUMOylated PML That Is Protected from Degradation by Herpes Simplex Virus 1 ICP0.J Virol. 2018 Nov 12;92(23):e01452-18. doi: 10.1128/JVI.01452-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30258013 Free PMC article.
-
Analysis of the functional interchange between the IE1 and pp71 proteins of human cytomegalovirus and ICP0 of herpes simplex virus 1.J Virol. 2015 Mar;89(6):3062-75. doi: 10.1128/JVI.03480-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552717 Free PMC article.
-
Stimulation of the Replication of ICP0-Null Mutant Herpes Simplex Virus 1 and pp71-Deficient Human Cytomegalovirus by Epstein-Barr Virus Tegument Protein BNRF1.J Virol. 2016 Oct 14;90(21):9664-9673. doi: 10.1128/JVI.01224-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535048 Free PMC article.
-
The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1.Protein Cell. 2012 May;3(5):372-82. doi: 10.1007/s13238-012-2021-x. Epub 2012 Apr 28. Protein Cell. 2012. PMID: 22544561 Free PMC article. Review.
Cited by
-
A Tale of Usurpation and Subversion: SUMO-Dependent Integrity of Promyelocytic Leukemia Nuclear Bodies at the Crossroad of Infection and Immunity.Front Cell Dev Biol. 2021 Aug 27;9:696234. doi: 10.3389/fcell.2021.696234. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513832 Free PMC article. Review.
-
Host Intrinsic and Innate Intracellular Immunity During Herpes Simplex Virus Type 1 (HSV-1) Infection.Front Microbiol. 2019 Nov 8;10:2611. doi: 10.3389/fmicb.2019.02611. eCollection 2019. Front Microbiol. 2019. PMID: 31781083 Free PMC article. Review.
-
Interactome and Ubiquitinome Analyses Identify Functional Targets of Herpes Simplex Virus 1 Infected Cell Protein 0.Front Microbiol. 2022 Apr 18;13:856471. doi: 10.3389/fmicb.2022.856471. eCollection 2022. Front Microbiol. 2022. PMID: 35516420 Free PMC article.
-
The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
-
Swine Promyelocytic Leukemia Isoform II Inhibits Pseudorabies Virus Infection by Suppressing Viral Gene Transcription in Promyelocytic Leukemia Nuclear Bodies.J Virol. 2020 Aug 31;94(18):e01197-20. doi: 10.1128/JVI.01197-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641476 Free PMC article.
References
-
- Knipe D, Howley P, Griffin D, Lamb R, Martin M, Roizman B, Strauss S. 2006. Fields Virology. Lippincott Williams and Wilkins, Philadelphia, PA.
-
- Weller S. 2011. Alphaherpesviruses. Caister Academic Press, Norfolk, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

