Molecular Determinants Directing HIV-1 Gag Assembly to Virus-Containing Compartments in Primary Macrophages
- PMID: 27440886
- PMCID: PMC5021390
- DOI: 10.1128/JVI.01004-16
Molecular Determinants Directing HIV-1 Gag Assembly to Virus-Containing Compartments in Primary Macrophages
Abstract
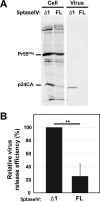
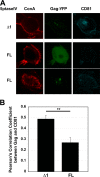
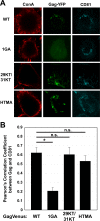
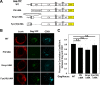
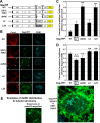
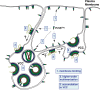
The subcellular sites of HIV-1 assembly, determined by the localization of the structural protein Gag, vary in a cell-type-dependent manner. In T cells and transformed cell lines used as model systems, HIV-1 assembles at the plasma membrane (PM). The binding and localization of HIV-1 Gag to the PM are mediated by the interaction between the matrix (MA) domain, specifically the highly basic region, and a PM-specific acidic phospholipid, phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]. In primary macrophages, prominent accumulation of assembling or assembled particles is found in the virus-containing compartments (VCCs), which largely consist of convoluted invaginations of the PM. To elucidate the molecular mechanism of HIV-1 Gag targeting to the VCCs, we examined the impact of overexpression of polyphosphoinositide 5-phosphatase IV (5ptaseIV), which depletes cellular PI(4,5)P2, in primary macrophages. We found that the VCC localization and virus release of HIV-1 are severely impaired upon 5ptaseIV overexpression, suggesting an important role for the MA-PI(4,5)P2 interaction in HIV-1 assembly in primary macrophages. However, our analysis of HIV-1 Gag derivatives with MA changes showed that this interaction contributes to Gag membrane binding but is dispensable for specific targeting of Gag to the VCCs per se We further determined that deletion of the NC domain abolishes VCC-specific localization of HIV-1 Gag. Notably, HIV-1 Gag localized efficiently to the VCCs when the NC domain was replaced with a leucine zipper dimerization motif that promotes Gag multimerization. Altogether, our data revealed that targeting of HIV-1 Gag to the VCCs requires NC-dependent multimerization.
Importance: In T cells and model cell lines, HIV-1 Gag localizes to the PM in a manner dependent on the MA-PI(4,5)P2 interaction. On the other hand, in primary macrophages, HIV-1 Gag localizes to convoluted intracellular membrane structures termed virus-containing compartments (VCCs). Although these compartments have been known for decades, and despite the implication of viruses in VCCs being involved in virus reservoir maintenance and spread, the viral determinant(s) that promotes Gag targeting to VCCs is unknown. In this study, we found that the MA-PI(4,5)P2 interaction facilitates efficient Gag membrane binding in macrophages but is not essential for Gag targeting to VCCs. Rather, our results revealed that NC-dependent multimerization promotes VCC targeting. Our findings highlight the differential roles played by MA and NC in HIV-1 Gag membrane binding and targeting and suggest a multimerization-dependent mechanism for Gag trafficking in primary macrophages similar to that for Gag localization to uropods in polarized T cells.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Relationships between MA-RNA Binding in Cells and Suppression of HIV-1 Gag Mislocalization to Intracellular Membranes.J Virol. 2019 Nov 13;93(23):e00756-19. doi: 10.1128/JVI.00756-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511376 Free PMC article.
-
Membrane binding and subcellular localization of retroviral Gag proteins are differentially regulated by MA interactions with phosphatidylinositol-(4,5)-bisphosphate and RNA.mBio. 2014 Dec 9;5(6):e02202. doi: 10.1128/mBio.02202-14. mBio. 2014. PMID: 25491356 Free PMC article.
-
Gag localization and virus-like particle release mediated by the matrix domain of human T-lymphotropic virus type 1 Gag are less dependent on phosphatidylinositol-(4,5)-bisphosphate than those mediated by the matrix domain of HIV-1 Gag.J Virol. 2011 Apr;85(8):3802-10. doi: 10.1128/JVI.02383-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289126 Free PMC article.
-
Relationship between HIV-1 Gag Multimerization and Membrane Binding.Viruses. 2022 Mar 16;14(3):622. doi: 10.3390/v14030622. Viruses. 2022. PMID: 35337029 Free PMC article. Review.
-
Roles played by acidic lipids in HIV-1 Gag membrane binding.Virus Res. 2014 Nov 26;193:108-15. doi: 10.1016/j.virusres.2014.06.015. Epub 2014 Jul 3. Virus Res. 2014. PMID: 24998886 Free PMC article. Review.
Cited by
-
Relationships between MA-RNA Binding in Cells and Suppression of HIV-1 Gag Mislocalization to Intracellular Membranes.J Virol. 2019 Nov 13;93(23):e00756-19. doi: 10.1128/JVI.00756-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511376 Free PMC article.
-
Myeloid Cell Interaction with HIV: A Complex Relationship.Front Immunol. 2017 Nov 30;8:1698. doi: 10.3389/fimmu.2017.01698. eCollection 2017. Front Immunol. 2017. PMID: 29250073 Free PMC article. Review.
-
Roles of Virion-Incorporated CD162 (PSGL-1), CD43, and CD44 in HIV-1 Infection of T Cells.Viruses. 2021 Sep 26;13(10):1935. doi: 10.3390/v13101935. Viruses. 2021. PMID: 34696365 Free PMC article. Review.
-
HIV-1 promotes ubiquitination of the amyloidogenic C-terminal fragment of APP to support viral replication.Nat Commun. 2023 Jul 15;14(1):4227. doi: 10.1038/s41467-023-40000-x. Nat Commun. 2023. PMID: 37454116 Free PMC article.
-
Type I Phosphatidylinositol-4-Phosphate 5-Kinases α and γ Play a Key Role in Targeting HIV-1 Pr55Gag to the Plasma Membrane.J Virol. 2020 Jul 1;94(14):e00189-20. doi: 10.1128/JVI.00189-20. Print 2020 Jul 1. J Virol. 2020. PMID: 32376619 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

