4-Hydroxyestradiol induces mammary epithelial cell transformation through Nrf2-mediated heme oxygenase-1 overexpression
- PMID: 27438141
- PMCID: PMC5352084
- DOI: 10.18632/oncotarget.10516
4-Hydroxyestradiol induces mammary epithelial cell transformation through Nrf2-mediated heme oxygenase-1 overexpression
Erratum in
-
Correction: 4-Hydroxyestradiol induces mammary epithelial cell transformation through Nrf2-mediated heme oxygenase-1 overexpression.Oncotarget. 2019 Feb 8;10(12):1266. doi: 10.18632/oncotarget.26681. eCollection 2019 Feb 8. Oncotarget. 2019. PMID: 30815229 Free PMC article.
-
Correction: Cancer therapeutic approach based on conformational stabilization of mutant p53 protein by small peptides.Oncotarget. 2019 May 7;10(34):3203-3206. doi: 10.18632/oncotarget.26921. eCollection 2019 May 7. Oncotarget. 2019. PMID: 31191814 Free PMC article.
Abstract
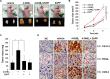
Estrogen (17β-estradiol, E2) undergoes oxidative metabolism by CYP1B1 to form 4-hydroxyestradiol (4-OHE2), a putative carcinogenic metabolite of estrogen. Our previous study showed that 4-OHE2-induced production of reactive oxygen species contributed to neoplastic transformation of human breast epithelial (MCF-10A) cells. In this study, 4-OHE2, but not E2, increased the expression of heme oxygenase-1 (HO-1), a sensor and regulator of oxidative stress, in MCF-10A cells. Silencing the HO-1 gene in MCF-10A cells suppressed 4-OHE2-induced cell proliferation and transformation. In addition, subcutaneous administration of 4-OHE2 markedly enhanced the growth of the MDA-MB-231 human breast cancer xenografts, which was retarded by zinc protoporphyrin, a pharmacological inhibitor of HO-1. 4-OHE2-induced HO-1 expression was mediated by NF-E2-related factor 2 (Nrf2). We speculate that an electrophilic quinone formed as a consequence of oxidation of 4-OHE2 binds directly to Kelch-like ECH-associated protein 1 (Keap1), an inhibitory protein that sequesters Nrf2 in the cytoplasm. This will diminish association between Nrf2 and Keap1. 4-OHE2 failed to interrupt the interaction between Keap1 and Nrf2 and to induce HO-1 expression in Keap1-C273S or C288S mutant cells. Lano-LC-ESI-MS/MS analysis in MCF-10A-Keap1-WT cells which were treated with 4-OHE2 revealed that the peptide fragment containing Cys288 gained a molecular mass of 287.15 Da, equivalent to the addition of a single molecule of 4-OHE2-derived ortho-quinones.
Keywords: 4-hydroxyestradiol; Nrf2; breast cancer; catechol estrogen; heme oxygenase-1.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures





Similar articles
-
Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling.Arch Biochem Biophys. 2010 Sep 1;501(1):142-50. doi: 10.1016/j.abb.2010.06.011. Epub 2010 Jun 15. Arch Biochem Biophys. 2010. PMID: 20558128
-
4-Hydroxyestradiol induces oxidative stress and apoptosis in human mammary epithelial cells: possible protection by NF-kappaB and ERK/MAPK.Toxicol Appl Pharmacol. 2005 Oct 1;208(1):46-56. doi: 10.1016/j.taap.2005.01.010. Toxicol Appl Pharmacol. 2005. PMID: 15901486
-
Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells.Int J Cancer. 2006 Apr 15;118(8):1862-8. doi: 10.1002/ijc.21590. Int J Cancer. 2006. PMID: 16287077
-
Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention.Biochim Biophys Acta. 2006 Aug;1766(1):63-78. doi: 10.1016/j.bbcan.2006.03.001. Epub 2006 Apr 19. Biochim Biophys Acta. 2006. PMID: 16675129 Review.
-
Synergistic Interaction Between Heme Oxygenase (HO) and Nuclear-Factor E2- Related Factor-2 (Nrf2) against Oxidative Stress in Cardiovascular Related Diseases.Curr Pharm Des. 2017;23(10):1465-1470. doi: 10.2174/1381612823666170113153818. Curr Pharm Des. 2017. PMID: 28088909 Review.
Cited by
-
Red Clover Aryl Hydrocarbon Receptor (AhR) and Estrogen Receptor (ER) Agonists Enhance Genotoxic Estrogen Metabolism.Chem Res Toxicol. 2017 Nov 20;30(11):2084-2092. doi: 10.1021/acs.chemrestox.7b00237. Epub 2017 Oct 19. Chem Res Toxicol. 2017. PMID: 28985473 Free PMC article.
-
Cytoprotective effects of mild plasma-activated medium against oxidative stress in human skin fibroblasts.Sci Rep. 2017 Feb 7;7:42208. doi: 10.1038/srep42208. Sci Rep. 2017. PMID: 28169359 Free PMC article.
-
Redox Signaling by Reactive Electrophiles and Oxidants.Chem Rev. 2018 Sep 26;118(18):8798-8888. doi: 10.1021/acs.chemrev.7b00698. Epub 2018 Aug 27. Chem Rev. 2018. PMID: 30148624 Free PMC article. Review.
-
Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility.Reprod Biol Endocrinol. 2019 Jan 6;17(1):4. doi: 10.1186/s12958-018-0449-4. Reprod Biol Endocrinol. 2019. PMID: 30611299 Free PMC article. Review.
-
Docosahexaenoic Acid Induces Expression of Heme Oxygenase-1 and NAD(P)H:quinone Oxidoreductase through Activation of Nrf2 in Human Mammary Epithelial Cells.Molecules. 2017 Jun 10;22(6):969. doi: 10.3390/molecules22060969. Molecules. 2017. PMID: 28604588 Free PMC article.
References
-
- Castagnetta LA, Granata OM, Traina A, Ravazzolo B, Amoroso M, Miele M, Bellavia V, Agostara B, Carruba G. Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin Cancer Res. 2002;8:3146–3155. - PubMed
-
- Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, Higginbotham SM, Cavalieri EL. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. - PubMed
-
- Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

