Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles
- PMID: 27435629
- PMCID: PMC4951696
- DOI: 10.1038/srep29418
Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles
Abstract
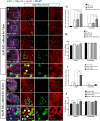
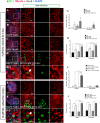
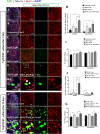
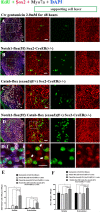
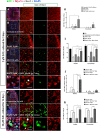
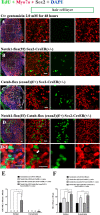
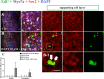
This work sought to determine the crosstalk between the Notch and Wnt signaling pathways in regulating supporting cell (SC) proliferation and hair cell (HC) regeneration in mouse utricles. We cultured postnatal day (P)3 and P60 mouse utricles, damaged the HCs with gentamicin, and treated the utricles with the γ-secretase inhibitor DAPT to inhibit the Notch pathway and with the Wnt agonist QS11 to active the Wnt pathway. We also used Sox2-CreER, Notch1-flox (exon 1), and Catnb-flox (exon 3) transgenic mice to knock out the Notch pathway and activate the Wnt pathway in Sox2+ SCs. Notch inhibition alone increased SC proliferation and HC number in both undamaged and damaged utricles. Wnt activation alone promoted SC proliferation, but the HC number was not significantly increased. Here we demonstrated the cumulative effects of Notch inhibition and Wnt activation in regulating SC proliferation and HC regeneration. Simultaneously inhibiting Notch and overexpressing Wnt led to significantly greater SC proliferation and greater numbers of HCs than manipulating either pathway alone. Similar results were observed in the transgenic mice. This study suggests that the combination of Notch inhibition and Wnt activation can significantly promote SC proliferation and increase the number of regenerated HCs in mouse utricle.
Figures
Similar articles
-
Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea.Oncotarget. 2016 Oct 11;7(41):66754-66768. doi: 10.18632/oncotarget.11479. Oncotarget. 2016. PMID: 27564256 Free PMC article.
-
The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea.Cell Tissue Res. 2021 Nov;386(2):281-296. doi: 10.1007/s00441-021-03493-w. Epub 2021 Jul 5. Cell Tissue Res. 2021. PMID: 34223978 Free PMC article.
-
Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea.J Neurosci. 2016 Aug 17;36(33):8734-45. doi: 10.1523/JNEUROSCI.0060-16.2016. J Neurosci. 2016. PMID: 27535918 Free PMC article.
-
Role of Wnt and Notch signaling in regulating hair cell regeneration in the cochlea.Front Med. 2016 Sep;10(3):237-49. doi: 10.1007/s11684-016-0464-9. Epub 2016 Sep 7. Front Med. 2016. PMID: 27527363 Review.
-
Signaling in the Auditory System: Implications in Hair Cell Regeneration and Hearing Function.J Cell Physiol. 2017 Oct;232(10):2710-2721. doi: 10.1002/jcp.25695. Epub 2017 Mar 29. J Cell Physiol. 2017. PMID: 27869308 Review.
Cited by
-
Single-Cell Sequencing Applications in the Inner Ear.Front Cell Dev Biol. 2021 Feb 12;9:637779. doi: 10.3389/fcell.2021.637779. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644075 Free PMC article. Review.
-
The interaction of Notch and Wnt signaling pathways in vertebrate regeneration.Cell Regen. 2021 Apr 1;10(1):11. doi: 10.1186/s13619-020-00072-2. Cell Regen. 2021. PMID: 33791915 Free PMC article. Review.
-
Hair cell regeneration from inner ear progenitors in the mammalian cochlea.Am J Stem Cells. 2020 Jun 15;9(3):25-35. eCollection 2020. Am J Stem Cells. 2020. PMID: 32699655 Free PMC article. Review.
-
Kölliker's organ-supporting cells and cochlear auditory development.Front Mol Neurosci. 2022 Oct 11;15:1031989. doi: 10.3389/fnmol.2022.1031989. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36304996 Free PMC article. Review.
-
Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea.Cell Mol Life Sci. 2020 Apr;77(7):1401-1419. doi: 10.1007/s00018-019-03291-2. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485717 Free PMC article.
References
-
- Dye B. J., Frank T. C., Newlands S. D. & Dickman J. D. Distribution and time course of hair cell regeneration in the pigeon utricle. Hear Res 133, 17–26 (1999). - PubMed
-
- Weisleder P. & Rubel E. W. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. The Journal of comparative neurology 331, 97–110 (1993). - PubMed
-
- Cafaro J., Lee G. S. & Stone J. S. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn 236, 156–170 (2007). - PubMed
-
- Stone J. S. & Cotanche D. A. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol 51, 633–647 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

