Intratumoral Infection with Murine Cytomegalovirus Synergizes with PD-L1 Blockade to Clear Melanoma Lesions and Induce Long-term Immunity
- PMID: 27434584
- PMCID: PMC5023369
- DOI: 10.1038/mt.2016.121
Intratumoral Infection with Murine Cytomegalovirus Synergizes with PD-L1 Blockade to Clear Melanoma Lesions and Induce Long-term Immunity
Abstract
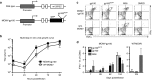
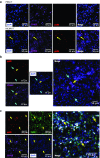
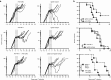
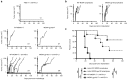
Cytomegalovirus is an attractive cancer vaccine platform because it induces strong, functional CD8(+) T-cell responses that accumulate over time and migrate into most tissues. To explore this, we used murine cytomegalovirus expressing a modified gp100 melanoma antigen. Therapeutic vaccination by the intraperitoneal and intradermal routes induced tumor infiltrating gp100-specific CD8(+) T-cells, but provided minimal benefit for subcutaneous lesions. In contrast, intratumoral infection of established tumor nodules greatly inhibited tumor growth and improved overall survival in a CD8(+) T-cell-dependent manner, even in mice previously infected with murine cytomegalovirus. Although murine cytomegalovirus could infect and kill B16F0s in vitro, infection was restricted to tumor-associated macrophages in vivo. Surprisingly, the presence of a tumor antigen in the virus only slightly increased the efficacy of intratumoral infection and tumor-specific CD8(+) T-cells in the tumor remained dysfunctional. Importantly, combining intratumoral murine cytomegalovirus infection with anti-PD-L1 therapy was synergistic, resulting in tumor clearance from over half of the mice and subsequent protection against tumor challenge. Thus, while a murine cytomegalovirus-based vaccine was poorly effective against established subcutaneous tumors, direct infection of tumor nodules unexpectedly delayed tumor growth and synergized with immune checkpoint blockade to promote tumor clearance and long-term protection.
Figures



Similar articles
-
In situ delivery of iPSC-derived dendritic cells with local radiotherapy generates systemic antitumor immunity and potentiates PD-L1 blockade in preclinical poorly immunogenic tumor models.J Immunother Cancer. 2021 May;9(5):e002432. doi: 10.1136/jitc-2021-002432. J Immunother Cancer. 2021. PMID: 34049930 Free PMC article.
-
Improved tumor immunity using anti-tyrosinase related protein-1 monoclonal antibody combined with DNA vaccines in murine melanoma.Cancer Res. 2008 Dec 1;68(23):9884-91. doi: 10.1158/0008-5472.CAN-08-2233. Cancer Res. 2008. PMID: 19047169 Free PMC article.
-
Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-cell Response and Protects Mice from Melanoma.Cancer Immunol Res. 2015 May;3(5):536-46. doi: 10.1158/2326-6066.CIR-14-0044. Epub 2015 Jan 29. Cancer Immunol Res. 2015. PMID: 25633711
-
Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity.Hum Vaccin Immunother. 2017 Aug 3;13(8):1778-1785. doi: 10.1080/21645515.2017.1331795. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28604162 Free PMC article. Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Transcriptional signature of durable effector T cells elicited by a replication defective HCMV vaccine.NPJ Vaccines. 2024 Apr 1;9(1):70. doi: 10.1038/s41541-024-00860-w. NPJ Vaccines. 2024. PMID: 38561339 Free PMC article.
-
FcγRI expression on macrophages is required for antibody-mediated tumor protection by cytomegalovirus-based vaccines.Oncotarget. 2018 Jun 29;9(50):29392-29402. doi: 10.18632/oncotarget.25630. eCollection 2018 Jun 29. Oncotarget. 2018. PMID: 30034625 Free PMC article.
-
From Vaccine Vector to Oncomodulation: Understanding the Complex Interplay between CMV and Cancer.Vaccines (Basel). 2019 Jul 9;7(3):62. doi: 10.3390/vaccines7030062. Vaccines (Basel). 2019. PMID: 31323930 Free PMC article. Review.
-
Vaccine Vectors Harnessing the Power of Cytomegaloviruses.Vaccines (Basel). 2019 Oct 17;7(4):152. doi: 10.3390/vaccines7040152. Vaccines (Basel). 2019. PMID: 31627457 Free PMC article. Review.
-
Exploring the Potential of Cytomegalovirus-Based Vectors: A Review.Viruses. 2023 Oct 2;15(10):2043. doi: 10.3390/v15102043. Viruses. 2023. PMID: 37896820 Free PMC article. Review.
References
-
- Schreiber, RD, Old, LJ and Smyth, MJ (2011). Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331: 1565–1570. - PubMed
-
- Wherry, E. J. (2011). T cell exhaustion. Nature Immunol 131: 492–499. - PubMed
-
- Azimi, F, Scolyer, RA, Rumcheva, P, Moncrieff, M, Murali, R, McCarthy, SW et al. (2012). Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30: 2678–2683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

