Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization
- PMID: 27434311
- PMCID: PMC4951126
- DOI: 10.1371/journal.ppat.1005742
Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization
Erratum in
-
Correction: Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization.PLoS Pathog. 2017 Sep 25;13(9):e1006641. doi: 10.1371/journal.ppat.1006641. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28945784 Free PMC article.
Abstract
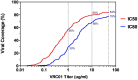
The development of biomedical interventions to reduce acquisition of HIV-1 infection remains a global priority, however their potential effectiveness is challenged by very high HIV-1 envelope diversity. Two large prophylactic trials in high incidence, clade C epidemic regions in southern Africa are imminent; passive administration of the monoclonal antibody VRC01, and active immunization with a clade C modified RV144-like vaccines. We have created a large representative panel of C clade viruses to enable assessment of antibody responses to vaccines and natural infection in Southern Africa, and we investigated the genotypic and neutralization properties of recently transmitted clade C viruses to determine how viral diversity impacted antibody recognition. We further explore the implications of these findings for the potential effectiveness of these trials. A panel of 200 HIV-1 Envelope pseudoviruses was constructed from clade C viruses collected within the first 100 days following infection. Viruses collected pre-seroconversion were significantly more resistant to serum neutralization compared to post-seroconversion viruses (p = 0.001). Over 13 years of the study as the epidemic matured, HIV-1 diversified (p = 0.0009) and became more neutralization resistant to monoclonal antibodies VRC01, PG9 and 4E10. When tested at therapeutic levels (10ug/ml), VRC01 only neutralized 80% of viruses in the panel, although it did exhibit potent neutralization activity against sensitive viruses (IC50 titres of 0.42 μg/ml). The Gp120 amino acid similarity between the clade C panel and candidate C-clade vaccine protein boosts (Ce1086 and TV1) was 77%, which is 8% more distant than between CRF01_AE viruses and the RV144 CRF01_AE immunogen. Furthermore, two vaccine signature sites, K169 in V2 and I307 in V3, associated with reduced infection risk in RV144, occurred less frequently in clade C panel viruses than in CRF01_AE viruses from Thailand. Increased resistance of pre-seroconversion viruses and evidence of antigenic drift highlights the value of using panels of very recently transmitted viruses and suggests that interventions may need to be modified over time to track the changing epidemic. Furthermore, high divergence such as that observed in the older clade C epidemic in southern Africa may impact vaccine efficacy, although the correlates of infection risk are yet to be defined in the clade C setting. Findings from this study of acute/early clade C viruses will aid vaccine development, and enable identification of new broad and potent antibodies to combat the HIV-1 C-clade epidemic in southern Africa.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures



Similar articles
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Anti-V3/Glycan and Anti-MPER Neutralizing Antibodies, but Not Anti-V2/Glycan Site Antibodies, Are Strongly Associated with Greater Anti-HIV-1 Neutralization Breadth and Potency.J Virol. 2015 May;89(10):5264-75. doi: 10.1128/JVI.00129-15. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673728 Free PMC article.
-
Progress in HIV vaccine development.Hum Vaccin Immunother. 2017 May 4;13(5):1018-1030. doi: 10.1080/21645515.2016.1276138. Epub 2017 Mar 10. Hum Vaccin Immunother. 2017. PMID: 28281871 Free PMC article. Review.
-
Prospects for a globally effective HIV-1 vaccine.Vaccine. 2015 Nov 27;33 Suppl 4:D4-12. doi: 10.1016/j.vaccine.2015.03.059. Epub 2015 Jun 20. Vaccine. 2015. PMID: 26100921 Review.
Cited by
-
Geospatial HIV-1 subtype C gp120 sequence diversity and its predicted impact on broadly neutralizing antibody sensitivity.PLoS One. 2021 May 24;16(5):e0251969. doi: 10.1371/journal.pone.0251969. eCollection 2021. PLoS One. 2021. PMID: 34029329 Free PMC article.
-
Physiotherapists' awareness of risk of bone demineralisation and falls in people living with HIV: a qualitative study.BMC Health Serv Res. 2021 Apr 13;21(1):333. doi: 10.1186/s12913-021-06343-1. BMC Health Serv Res. 2021. PMID: 33849529 Free PMC article.
-
Subtle Longitudinal Alterations in Env Sequence Potentiate Differences in Sensitivity to Broadly Neutralizing Antibodies following Acute HIV-1 Subtype C Infection.J Virol. 2022 Dec 21;96(24):e0127022. doi: 10.1128/jvi.01270-22. Epub 2022 Dec 1. J Virol. 2022. PMID: 36453881 Free PMC article.
-
Impact of HIV-1 Diversity on Its Sensitivity to Neutralization.Vaccines (Basel). 2019 Jul 25;7(3):74. doi: 10.3390/vaccines7030074. Vaccines (Basel). 2019. PMID: 31349655 Free PMC article. Review.
-
Application of the SLAPNAP statistical learning tool to broadly neutralizing antibody HIV prevention research.iScience. 2023 Aug 9;26(9):107595. doi: 10.1016/j.isci.2023.107595. eCollection 2023 Sep 15. iScience. 2023. PMID: 37654470 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

