Sar1 localizes at the rims of COPII-coated membranes in vivo
- PMID: 27432890
- PMCID: PMC5047700
- DOI: 10.1242/jcs.189423
Sar1 localizes at the rims of COPII-coated membranes in vivo
Abstract
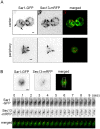
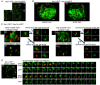
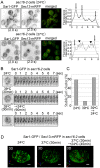
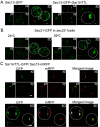
The Sar1 GTPase controls coat assembly on coat protein complex II (COPII)-coated vesicles, which mediate protein transport from the endoplasmic reticulum (ER) to the Golgi. The GTP-bound form of Sar1, activated by the ER-localized guanine nucleotide exchange factor (GEF) Sec12, associates with the ER membrane. GTP hydrolysis by Sar1, stimulated by the COPII-vesicle-localized GTPase-activating protein (GAP) Sec23, in turn causes Sar1 to dissociate from the membrane. Thus, Sar1 is cycled between active and inactive states, and on and off vesicle membranes, but its precise spatiotemporal regulation remains unknown. Here, we examined Sar1 localization on COPII-coated membranes in living Saccharomyces cerevisiae cells. Two-dimensional (2D) observation demonstrated that Sar1 showed modest accumulation around the ER exit sites (ERES) in a manner that was dependent on Sec16 function. Detailed three-dimensional (3D) observation further demonstrated that Sar1 localized at the rims of the COPII-coated membranes, but was excluded from the rest of the COPII membranes. Additionally, a GTP-locked form of Sar1 induced abnormally enlarged COPII-coated structures and covered the entirety of these structures. These results suggested that the reversible membrane association of Sar1 GTPase leads to its localization being restricted to the rims of COPII-coated membranes in vivo.
Keywords: COPII-coated vesicle; ER; GTP hydrolysis; Sar1; Sec16.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites.Mol Biol Cell. 2012 Aug;23(15):2930-42. doi: 10.1091/mbc.E12-05-0356. Epub 2012 Jun 6. Mol Biol Cell. 2012. PMID: 22675024 Free PMC article.
-
Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission.J Cell Biol. 2005 Dec 19;171(6):919-24. doi: 10.1083/jcb.200509095. Epub 2005 Dec 12. J Cell Biol. 2005. PMID: 16344311 Free PMC article.
-
Sed4p stimulates Sar1p GTP hydrolysis and promotes limited coat disassembly.Traffic. 2011 May;12(5):591-9. doi: 10.1111/j.1600-0854.2011.01173.x. Epub 2011 Feb 25. Traffic. 2011. PMID: 21291503
-
The small GTPase Sar1, control centre of COPII trafficking.FEBS Lett. 2023 Mar;597(6):865-882. doi: 10.1002/1873-3468.14595. Epub 2023 Feb 20. FEBS Lett. 2023. PMID: 36737236 Review.
-
COPII coat assembly and selective export from the endoplasmic reticulum.J Biochem. 2004 Dec;136(6):755-60. doi: 10.1093/jb/mvh184. J Biochem. 2004. PMID: 15671485 Review.
Cited by
-
A simple supported tubulated bilayer system for evaluating protein-mediated membrane remodeling.Chem Phys Lipids. 2018 Sep;215:18-28. doi: 10.1016/j.chemphyslip.2018.06.002. Epub 2018 Jul 22. Chem Phys Lipids. 2018. PMID: 30012406 Free PMC article.
-
Regulation of the Sar1 GTPase Cycle Is Necessary for Large Cargo Secretion from the Endoplasmic Reticulum.Front Cell Dev Biol. 2017 Aug 23;5:75. doi: 10.3389/fcell.2017.00075. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28879181 Free PMC article. Review.
-
Super resolution live imaging: The key for unveiling the true dynamics of membrane traffic around the Golgi apparatus in plant cells.Front Plant Sci. 2022 Dec 22;13:1100757. doi: 10.3389/fpls.2022.1100757. eCollection 2022. Front Plant Sci. 2022. PMID: 36618665 Free PMC article. Review.
-
Live-cell Imaging by Super-resolution Confocal Live Imaging Microscopy (SCLIM): Simultaneous Three-color and Four-dimensional Live Cell Imaging with High Space and Time Resolution.Bio Protoc. 2020 Sep 5;10(17):e3732. doi: 10.21769/BioProtoc.3732. eCollection 2020 Sep 5. Bio Protoc. 2020. PMID: 33659393 Free PMC article.
-
COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers.J Cell Biol. 2021 Jun 7;220(6):e201907224. doi: 10.1083/jcb.201907224. J Cell Biol. 2021. PMID: 33852719 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

