Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation?
- PMID: 27425629
- PMCID: PMC4947958
- DOI: 10.1038/srep29949
Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation?
Abstract
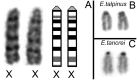
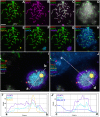
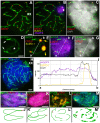
Most mammalian species have heteromorphic sex chromosomes in males, except for a few enigmatic groups such as the mole voles Ellobius, which do not have the Y chromosome and Sry gene. The Ellobius (XX ♀♂) system of sex chromosomes has no analogues among other animals. The structure and meiotic behaviour of the two X chromosomes were investigated for males of the sibling species Ellobius talpinus and Ellobius tancrei. Their sex chromosomes, despite their identical G-structure, demonstrate short synaptic fragments and crossover-associated MLH1 foci in both telomeric regions only. The chromatin undergoes modifications in the meiotic sex chromosomes. SUMO-1 marks a small nucleolus-like body of the meiotic XX. ATR and ubiH2A are localized in the asynaptic area and the histone γH2AFX covers the entire XX bivalent. The distribution of some markers of chromatin inactivation differentiates sex chromosomes of mole voles from those of other mammals. Sex chromosomes of both studied species have identical recombination and meiotic inactivation patterns. In Ellobius, similar chromosome morphology masks the functional heteromorphism of the male sex chromosomes, which can be seen at meiosis.
Figures

Similar articles
-
Sex differences in the meiotic behavior of an XX sex chromosome pair in males and females of the mole vole Ellobius tancrei: turning an X into a Y chromosome?Chromosoma. 2021 Sep;130(2-3):113-131. doi: 10.1007/s00412-021-00755-y. Epub 2021 Apr 6. Chromosoma. 2021. PMID: 33825031
-
Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes.Int J Mol Sci. 2021 Feb 17;22(4):1969. doi: 10.3390/ijms22041969. Int J Mol Sci. 2021. PMID: 33671248 Free PMC article.
-
Genomes of Ellobius species provide insight into the evolutionary dynamics of mammalian sex chromosomes.Genome Res. 2016 Sep;26(9):1202-10. doi: 10.1101/gr.201665.115. Epub 2016 Aug 10. Genome Res. 2016. PMID: 27510564 Free PMC article.
-
[Meiotic inactivation of sex chromosomes in mammals].Genetika. 2010 Apr;46(4):437-47. Genetika. 2010. PMID: 20536013 Review. Russian.
-
Ellobius lutescens: sex determination and sex chromosome.Sex Dev. 2007;1(4):211-21. doi: 10.1159/000104771. Sex Dev. 2007. PMID: 18391532 Review.
Cited by
-
Sex differences in the meiotic behavior of an XX sex chromosome pair in males and females of the mole vole Ellobius tancrei: turning an X into a Y chromosome?Chromosoma. 2021 Sep;130(2-3):113-131. doi: 10.1007/s00412-021-00755-y. Epub 2021 Apr 6. Chromosoma. 2021. PMID: 33825031
-
Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination.Int J Mol Sci. 2020 Oct 15;21(20):7630. doi: 10.3390/ijms21207630. Int J Mol Sci. 2020. PMID: 33076404 Free PMC article.
-
Sex chromosome quadrivalents in oocytes of the African pygmy mouse Mus minutoides that harbors non-conventional sex chromosomes.Chromosoma. 2019 Sep;128(3):397-411. doi: 10.1007/s00412-019-00699-4. Epub 2019 Mar 27. Chromosoma. 2019. PMID: 30919035
-
Evolutionary rearrangements of X chromosomes in voles (Arvicolinae, Rodentia).Sci Rep. 2020 Aug 6;10(1):13235. doi: 10.1038/s41598-020-70226-4. Sci Rep. 2020. PMID: 32764633 Free PMC article.
-
Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes.Int J Mol Sci. 2021 Feb 17;22(4):1969. doi: 10.3390/ijms22041969. Int J Mol Sci. 2021. PMID: 33671248 Free PMC article.
References
-
- Ohno S. Sex chromosomes and sex-linked genes. (Springer-Verlag, 1967).
-
- Сharlesworth D., Charlesworth B. & Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 (2005). - PubMed
-
- Graves J. A. M. Sex chromosome specialization and degeneration in mammals. Cell 124, 901–914 (2006). - PubMed
-
- Rice W. R. Evolution of the Y sex chromosome in animals. Bioscience 46, 331–343 (1996).
-
- Sinclair A. H. et al.. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

