Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview
- PMID: 27423335
- PMCID: PMC4961276
- DOI: 10.12659/msm.899852
Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview
Abstract
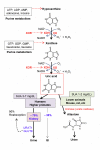
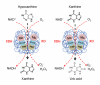
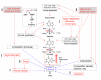
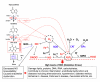
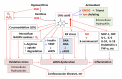
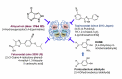
Uric acid is the final oxidation product of purine metabolism in humans. Xanthine oxidoreductase (XOR) catalyzes oxidative hydroxylation of hypoxanthine to xanthine to uric acid, accompanying the production of reactive oxygen species (ROS). Uric acid usually forms ions and salts known as urates and acid urates in serum. Clinically, overproduction or under-excretion of uric acid results in the elevated level of serum uric acid (SUA), termed hyperuricemia, which has long been established as the major etiologic factor in gout. Accordingly, urate-lowering drugs such as allopurinol, an XOR-inhibitor, are extensively used for the treatment of gout. In recent years, the prevalence of hyperuricemia has significantly increased and more clinical investigations have confirmed that hyperuricemia is an independent risk factor for cardiovascular disease, hypertension, diabetes, and many other diseases. Urate-lowering therapy may also play a critical role in the management of these diseases. However, current XOR-inhibitor drugs such as allopurinol and febuxostat may have significant adverse effects. Therefore, there has been great effort to develop new XOR-inhibitor drugs with less or no toxicity for the long-term treatment or prevention of these hyperuricemia-related diseases. In this review, we discuss the mechanism of uric acid homeostasis and alterations, updated prevalence, therapeutic outcomes, and molecular pathophysiology of hyperuricemia-related diseases. We also summarize current discoveries in the development of new XOR inhibitors.
Figures
Similar articles
-
The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors.Oxid Med Cell Longev. 2021 Mar 26;2021:1470380. doi: 10.1155/2021/1470380. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33854690 Free PMC article. Review.
-
Xanthine oxidoreductase and its inhibitors: relevance for gout.Clin Sci (Lond). 2016 Dec 1;130(23):2167-2180. doi: 10.1042/CS20160010. Clin Sci (Lond). 2016. PMID: 27798228 Review.
-
Identification of a novel xanthine oxidoreductase inhibitor for hyperuricemia treatment with high efficacy and safety profile.Biomed Pharmacother. 2024 Sep;178:117223. doi: 10.1016/j.biopha.2024.117223. Epub 2024 Aug 1. Biomed Pharmacother. 2024. PMID: 39094541
-
Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout.J Biol Inorg Chem. 2015 Mar;20(2):195-207. doi: 10.1007/s00775-014-1210-x. Epub 2014 Dec 12. J Biol Inorg Chem. 2015. PMID: 25501928 Free PMC article. Review.
-
New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity.Am J Physiol Endocrinol Metab. 2020 Nov 1;319(5):E827-E834. doi: 10.1152/ajpendo.00378.2020. Epub 2020 Sep 7. Am J Physiol Endocrinol Metab. 2020. PMID: 32893671 Review.
Cited by
-
The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors.Oxid Med Cell Longev. 2021 Mar 26;2021:1470380. doi: 10.1155/2021/1470380. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33854690 Free PMC article. Review.
-
An Efficient Enzyme-Less Uric Acid Sensor Development Based on PbO-Doped NiO Nanocomposites.Biosensors (Basel). 2022 May 31;12(6):381. doi: 10.3390/bios12060381. Biosensors (Basel). 2022. PMID: 35735529 Free PMC article.
-
Efficacy and Safety of Dotinurad in Hyperuricemic Patients With or Without Gout: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Cureus. 2021 Apr 12;13(4):e14428. doi: 10.7759/cureus.14428. Cureus. 2021. PMID: 33996294 Free PMC article.
-
Phytoconstituent Profiles Associated with Relevant Antioxidant Potential and Variable Nutritive Effects of the Olive, Sweet Almond, and Black Mulberry Gemmotherapy Extracts.Antioxidants (Basel). 2023 Sep 4;12(9):1717. doi: 10.3390/antiox12091717. Antioxidants (Basel). 2023. PMID: 37760021 Free PMC article.
-
Serum uric acid: an independent risk factor for cardiovascular disease in Pakistani Punjabi patients.BMC Cardiovasc Disord. 2024 Oct 10;24(1):546. doi: 10.1186/s12872-024-04055-y. BMC Cardiovasc Disord. 2024. PMID: 39385070 Free PMC article.
References
-
- Galassi FM, Borghi C. A brief history of uric acid: from gout to cardiovascular risk factor. Eur J Intern Med. 2015;26:373. - PubMed
-
- Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: A systematic review and meta-analysis. Clin Chem Lab Med. 2016;54(1):7–15. - PubMed
-
- Borghi C, Desideri G. Urate-lowering drugs and prevention of cardiovascular disease: The emerging role of xanthine oxidase inhibition. Hypertension. 2016;67(3):496–98. - PubMed
-
- Chauhan M, Kumar R. A comprehensive review on bioactive fused heterocycles as purine-utilizing enzymes inhibitors. Med Chem Res. 2015;24:2259–82.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

