Is TMC1 the Hair Cell Mechanotransducer Channel?
- PMID: 27410728
- PMCID: PMC4945579
- DOI: 10.1016/j.bpj.2016.05.032
Is TMC1 the Hair Cell Mechanotransducer Channel?
Abstract
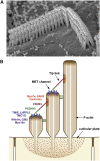
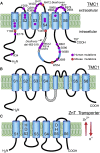
Transmembrane channel-like protein isoform-1 (TMC1) has emerged over the past five years as a prime contender for the mechano-electrical transducer (MET) channel in hair cells of the inner ear. TMC1 is thought to have a six-transmembrane domain structure reminiscent of some other ion-channel subunits, and is targeted to the tips of the stereocilia in the sensory hair bundle, where the MET channel is located. Moreover, there are TMC1 mutations linked to human deafness causing loss of conventional MET currents, hair cell degeneration, and deafness in mice. Finally, mutations of Tmc1 can alter the conductance and Ca(2+) selectivity of the MET channels. For several reasons though, it is unclear that TMC1 is indeed the MET channel pore: 1) in other animals or tissues, mutations of TMC family members do not directly affect cellular mechanosensitivity; 2) there are residual manifestations of mechanosensitivity in hair cells of mouse Tmc1:Tmc2 double knockouts; 3) there is so far no evidence that expression of mammalian Tmc1 generates a mechanically sensitive ion channel in the plasma membrane when expressed in heterologous cells; and 4) there are other proteins, such as TMIE and LHFPL5, which behave similarly to TMC1, their mutation also leading to loss of MET current and deafness. This review will present these disparate lines of evidence and describes recent work that addresses the role of TMC1.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The conductance and organization of the TMC1-containing mechanotransducer channel complex in auditory hair cells.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2210849119. doi: 10.1073/pnas.2210849119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191207 Free PMC article.
-
New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells.J Neurosci. 2021 May 19;41(20):4378-4391. doi: 10.1523/JNEUROSCI.2537-20.2021. Epub 2021 Apr 6. J Neurosci. 2021. PMID: 33824189 Free PMC article.
-
Function and Dysfunction of TMC Channels in Inner Ear Hair Cells.Cold Spring Harb Perspect Med. 2019 Oct 1;9(10):a033506. doi: 10.1101/cshperspect.a033506. Cold Spring Harb Perspect Med. 2019. PMID: 30291150 Free PMC article. Review.
-
The contribution of TMC1 to adaptation of mechanoelectrical transduction channels in cochlear outer hair cells.J Physiol. 2019 Dec;597(24):5949-5961. doi: 10.1113/JP278799. Epub 2019 Nov 12. J Physiol. 2019. PMID: 31633194 Free PMC article.
-
Mechanically Gated Ion Channels in Mammalian Hair Cells.Front Cell Neurosci. 2018 Apr 11;12:100. doi: 10.3389/fncel.2018.00100. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29755320 Free PMC article. Review.
Cited by
-
TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells.Neuron. 2018 Aug 22;99(4):736-753.e6. doi: 10.1016/j.neuron.2018.07.033. Neuron. 2018. PMID: 30138589 Free PMC article.
-
Insights into Electroreceptor Development and Evolution from Molecular Comparisons with Hair Cells.Integr Comp Biol. 2018 Aug 1;58(2):329-340. doi: 10.1093/icb/icy037. Integr Comp Biol. 2018. PMID: 29846597 Free PMC article.
-
Using Drosophila to study mechanisms of hereditary hearing loss.Dis Model Mech. 2018 May 31;11(6):dmm031492. doi: 10.1242/dmm.031492. Dis Model Mech. 2018. PMID: 29853544 Free PMC article. Review.
-
Over-expression of myosin7A in cochlear hair cells of circling mice.Lab Anim Res. 2017 Mar;33(1):1-7. doi: 10.5625/lar.2017.33.1.1. Epub 2017 Mar 27. Lab Anim Res. 2017. PMID: 28400833 Free PMC article.
-
Novel homozygous variants in the TMC1 and CDH23 genes cause autosomal recessive nonsyndromic hearing loss.Mol Genet Genomic Med. 2020 Dec;8(12):e1550. doi: 10.1002/mgg3.1550. Epub 2020 Nov 18. Mol Genet Genomic Med. 2020. PMID: 33205915 Free PMC article.
References
-
- Arnadóttir J., Chalfie M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010;39:111–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

