Synthetic low-density lipoprotein (sLDL) selectively delivers paclitaxel to tumor with low systemic toxicity
- PMID: 27409176
- PMCID: PMC5239495
- DOI: 10.18632/oncotarget.10493
Synthetic low-density lipoprotein (sLDL) selectively delivers paclitaxel to tumor with low systemic toxicity
Abstract
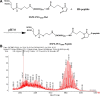
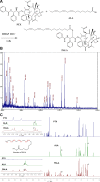
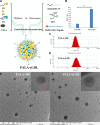
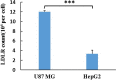
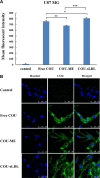
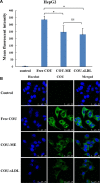
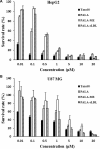
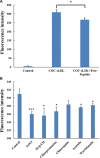
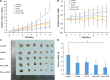
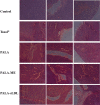
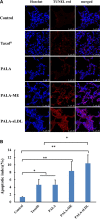
Low density lipoprotein (LDL), which is a principal carrier for the delivery of cholesterol, has been used as a great candidate for the delivery of drugs to tumor based on the great requirements for cholesterol of many cancer cells. Mimicking the structure and composition of LDL, we designed a synthetic low-density lipoprotein (sLDL) to encapsulate paclitaxel-alpha linolenic acid (PALA) for tumor therapy. The PALA loaded sLDL (PALA-sLDL) and PALA-loaded microemulsion (PALA-ME, without the binding domain for LDLR) displayed uniform sizes with high drug loading efficiency (> 90%). In vitro studies demonstrated PALA-sLDL exhibited enhanced cellular uptake capacity and better cytotoxicity to LDLR over-expressed U87 MG cells as compared to PALA-ME. The uptake mechanisms of PALA-sLDL were involved in a receptor mediated endocytosis and macropinocytosis. Furthermore, the in vivo biodistribution and tumor growth inhibition studies of PALA-sLDL were investigated in xenograft U87 MG tumor-bearing mice. The results showed that PALA-sLDL exhibited higher tumor accumulation than PALA-ME and superior tumor inhibition efficiency (72.1%) compared to Taxol® (51.2%) and PALA-ME (58.8%) but with lower toxicity. These studies suggested that sLDL is potential to be used as a valuable carrier for the selective delivery of anticancer drugs to tumor with low systemic toxicity.
Keywords: PTX-alpha linolenic acid (PALA); anti-tumor efficacy; biomimetic; low systemic toxicity; synthetic low-density lipoprotein (sLDL).
Conflict of interest statement
All the authors declare no conflicts of interests.
Figures

Similar articles
-
Improved safety and efficacy of a lipid emulsion loaded with a paclitaxel-cholesterol complex for the treatment of breast tumors.Oncol Rep. 2016 Jul;36(1):399-409. doi: 10.3892/or.2016.4787. Epub 2016 May 6. Oncol Rep. 2016. PMID: 27175803
-
Deoxycholic acid-modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy.Int J Pharm. 2014 Nov 20;475(1-2):60-8. doi: 10.1016/j.ijpharm.2014.08.037. Epub 2014 Aug 23. Int J Pharm. 2014. PMID: 25152167
-
Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect.J Control Release. 2010 Apr 2;143(1):136-42. doi: 10.1016/j.jconrel.2009.12.020. Epub 2010 Jan 7. J Control Release. 2010. PMID: 20056123
-
[Recent development of natural and reconstituted lipoprotein based nano drug delivery vehicles].Yao Xue Xue Bao. 2014 Jan;49(1):23-9. Yao Xue Xue Bao. 2014. PMID: 24783501 Review. Chinese.
-
Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells.Bioconjug Chem. 1994 Mar-Apr;5(2):105-13. doi: 10.1021/bc00026a002. Bioconjug Chem. 1994. PMID: 8031872 Review. No abstract available.
Cited by
-
Lipoprotein-based drug delivery.Adv Drug Deliv Rev. 2020;159:377-390. doi: 10.1016/j.addr.2020.08.003. Epub 2020 Aug 11. Adv Drug Deliv Rev. 2020. PMID: 32791075 Free PMC article. Review.
-
Biomimetics: reconstitution of low-density lipoprotein for targeted drug delivery and related theranostic applications.Chem Soc Rev. 2017 Dec 11;46(24):7668-7682. doi: 10.1039/c7cs00492c. Chem Soc Rev. 2017. PMID: 29104991 Free PMC article. Review.
-
Cell internalization of 7-ketocholesterol-containing nanoemulsion through LDL receptor reduces melanoma growth in vitro and in vivo: a preliminary report.Oncotarget. 2018 Feb 4;9(18):14160-14174. doi: 10.18632/oncotarget.24389. eCollection 2018 Mar 6. Oncotarget. 2018. PMID: 29581835 Free PMC article.
-
Synthetic lipoprotein as nano-material vehicle in the targeted drug delivery.Drug Deliv. 2017 Dec;24(sup1):16-21. doi: 10.1080/10717544.2017.1384518. Drug Deliv. 2017. PMID: 29069931 Free PMC article. Review.
-
Low-density lipoprotein nanomedicines: mechanisms of targeting, biology, and theranostic potential.Drug Deliv. 2021 Dec;28(1):408-421. doi: 10.1080/10717544.2021.1886199. Drug Deliv. 2021. PMID: 33594923 Free PMC article. Review.
References
-
- Howat S, Park B, Oh IS, Jin YW, Lee EK, Loake GJ. Paclitaxel: biosynthesis, production and future prospects. N Biotechnol. 2014;31:242–245. - PubMed
-
- Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. - PubMed
-
- Villano JL, Mehta D, Radhakrishnan L. Abraxane induced life-threatening toxicities with metastatic breast cancer and hepatic insufficiency. Invest New Drugs. 2006;24:455–456. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

