Marine Natural Products as Models to Circumvent Multidrug Resistance
- PMID: 27399665
- PMCID: PMC6273648
- DOI: 10.3390/molecules21070892
Marine Natural Products as Models to Circumvent Multidrug Resistance
Abstract
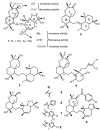
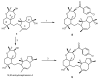
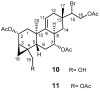
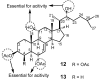
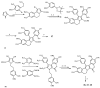
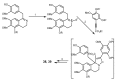
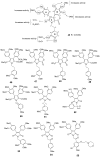
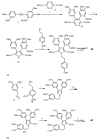
Multidrug resistance (MDR) to anticancer drugs is a serious health problem that in many cases leads to cancer treatment failure. The ATP binding cassette (ABC) transporter P-glycoprotein (P-gp), which leads to premature efflux of drugs from cancer cells, is often responsible for MDR. On the other hand, a strategy to search for modulators from natural products to overcome MDR had been in place during the last decades. However, Nature limits the amount of some natural products, which has led to the development of synthetic strategies to increase their availability. This review summarizes the research findings on marine natural products and derivatives, mainly alkaloids, polyoxygenated sterols, polyketides, terpenoids, diketopiperazines, and peptides, with P-gp inhibitory activity highlighting the established structure-activity relationships. The synthetic pathways for the total synthesis of the most promising members and analogs are also presented. It is expected that the data gathered during the last decades concerning their synthesis and MDR-inhibiting activities will help medicinal chemists develop potential drug candidates using marine natural products as models which can deliver new ABC transporter inhibitor scaffolds.
Keywords: ABC transporters; P-glycoprotein modulators; marine natural products; multidrug resistance (MDR); structure activity relationship (SAR); synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


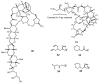
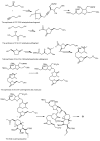
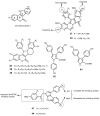
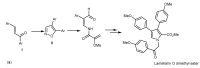


Similar articles
-
Multidrug resistance: retrospect and prospects in anti-cancer drug treatment.Curr Med Chem. 2006;13(16):1859-76. doi: 10.2174/092986706777585077. Curr Med Chem. 2006. PMID: 16842198 Review.
-
Overcoming Multidrug Resistance: Flavonoid and Terpenoid Nitrogen-Containing Derivatives as ABC Transporter Modulators.Molecules. 2020 Jul 24;25(15):3364. doi: 10.3390/molecules25153364. Molecules. 2020. PMID: 32722234 Free PMC article. Review.
-
Marine natural products as breast cancer resistance protein inhibitors.Mar Drugs. 2015 Apr 3;13(4):2010-29. doi: 10.3390/md13042010. Mar Drugs. 2015. PMID: 25854646 Free PMC article. Review.
-
β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function.Phytomedicine. 2016 Mar 15;23(3):316-23. doi: 10.1016/j.phymed.2016.01.008. Epub 2016 Feb 6. Phytomedicine. 2016. PMID: 26969385
-
Reversal of multidrug resistance by Marsdenia tenacissima and its main active ingredients polyoxypregnanes.J Ethnopharmacol. 2017 May 5;203:110-119. doi: 10.1016/j.jep.2017.03.051. Epub 2017 Mar 28. J Ethnopharmacol. 2017. PMID: 28363522
Cited by
-
Diketopiperazine Gels: New Horizons from the Self-Assembly of Cyclic Dipeptides.Molecules. 2021 Jun 3;26(11):3376. doi: 10.3390/molecules26113376. Molecules. 2021. PMID: 34204905 Free PMC article. Review.
-
Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug.Nutrients. 2022 Feb 13;14(4):786. doi: 10.3390/nu14040786. Nutrients. 2022. PMID: 35215436 Free PMC article.
-
Bioprospecting the microbiome of Red Sea Atlantis II brine pool for peptidases and biosynthetic genes with promising antibacterial activity.Microb Cell Fact. 2022 Jun 2;21(1):109. doi: 10.1186/s12934-022-01835-z. Microb Cell Fact. 2022. PMID: 35655185 Free PMC article.
-
Remodeling tumor microenvironment with natural products to overcome drug resistance.Front Immunol. 2022 Nov 10;13:1051998. doi: 10.3389/fimmu.2022.1051998. eCollection 2022. Front Immunol. 2022. PMID: 36439106 Free PMC article. Review.
-
Bioinformatics for Marine Products: An Overview of Resources, Bottlenecks, and Perspectives.Mar Drugs. 2019 Oct 11;17(10):576. doi: 10.3390/md17100576. Mar Drugs. 2019. PMID: 31614509 Free PMC article. Review.
References
-
- Cancer. [(accessed on 16 March 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/
-
- Che X.F., Nakajima Y., Sumizawa T., Ikeda R., Ren X.Q., Zheng C.L., Mukai M., Furukawa T., Haraguchi M., Gao H., et al. Reversal of p-glycoprotein mediated multidrug resistance by a newly synthesized 1,4-benzothiazipine derivative, jtv-519. Cancer Lett. 2002;187:111–119. doi: 10.1016/S0304-3835(02)00359-2. - DOI - PubMed
-
- Gustav L. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets. 2000;1:85–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

