Growing a whole porcine liver organ ex situ for six hours without red blood cells or hemoglobin
- PMID: 27398140
- PMCID: PMC4931151
Growing a whole porcine liver organ ex situ for six hours without red blood cells or hemoglobin
Abstract
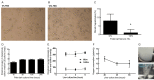
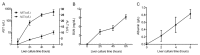
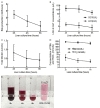
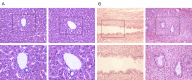
Liver transplantation is an effective approach to end-stage liver disease. Shortage of donor liver and increased waiting time for liver transplantation necessitate the development of an organ culture system by which livers can be cultured and maintained ex situ for a prolonged period of time. The aim of this work is to test whether cell culture condition in vitro could be used to culture whole livers ex situ without the use of erythrocytes. Twelve castrated male land race/farm young porcine livers were exposed to 30 min warm ischemia and 30 min cold perfusion. Livers were isolated and connected to an Ex situ liver culture system using a standard culture medium RPMI1640 supplied with 10% of fetal bovine serum and sufficient dissolved oxygen under a normothermic condition for 6 hours. Metabolic biomarkers, bile and urea production, hepatic cell viability and histology analysis of biopsies were examined and newly proliferated hepatic cells labeled by BrdU were analyzed after 6 hours ex situ culture. The results from biochemical assays and histology analysis indicate that livers after the organ culture still maintain the full function.
Conclusions: Our data demonstrate that the liver culture system established in this work can be used to culture whole livers ex situ in the absence of erythrocytes.
Keywords: 3D culture; BrdU histology analysis; BrdU proliferation assay; Ex situ liver culture; organ growing; oxygen carrier free; warm ischemia; without erythrocytes.
Figures



Similar articles
-
Biliary epithelial cells proliferate during oxygenated ex situ liver culture.Am J Transl Res. 2016 Sep 15;8(9):3955-3962. eCollection 2016. Am J Transl Res. 2016. PMID: 27725875 Free PMC article.
-
Determination of Minimal Hemoglobin Level Necessary for Normothermic Porcine Ex Situ Liver Perfusion.Transplantation. 2018 Aug;102(8):1284-1292. doi: 10.1097/TP.0000000000002272. Transplantation. 2018. PMID: 29757899
-
Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion.Transplantation. 2004 May 15;77(9):1328-32. doi: 10.1097/01.tp.0000119206.63326.56. Transplantation. 2004. PMID: 15167586
-
Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers.Int J Mol Sci. 2020 Dec 28;22(1):235. doi: 10.3390/ijms22010235. Int J Mol Sci. 2020. PMID: 33379394 Free PMC article. Review.
-
Normothermic ex-vivo liver perfusion: where do we stand and where to reach?Expert Rev Gastroenterol Hepatol. 2018 Oct;12(10):1045-1058. doi: 10.1080/17474124.2018.1505499. Epub 2018 Aug 20. Expert Rev Gastroenterol Hepatol. 2018. PMID: 30064278 Review.
Cited by
-
Biliary epithelial cells proliferate during oxygenated ex situ liver culture.Am J Transl Res. 2016 Sep 15;8(9):3955-3962. eCollection 2016. Am J Transl Res. 2016. PMID: 27725875 Free PMC article.
-
Control of Ischemia-Reperfusion Injury in Liver Transplantation: Potentials for Increasing the Donor Pool.Visc Med. 2018 Dec;34(6):444-448. doi: 10.1159/000493889. Epub 2018 Oct 30. Visc Med. 2018. PMID: 30675491 Free PMC article. Review.
-
Translational medical research and liver transplantation: systematic review.Transl Gastroenterol Hepatol. 2018 Nov 12;3:91. doi: 10.21037/tgh.2018.10.14. eCollection 2018. Transl Gastroenterol Hepatol. 2018. PMID: 30603727 Free PMC article. Review.
-
Magnetic-targeting of polyethylenimine-wrapped iron oxide nanoparticle labeled chondrocytes in a rabbit articular cartilage defect model.RSC Adv. 2018 Feb 16;8(14):7633-7640. doi: 10.1039/c7ra12039g. eCollection 2018 Feb 14. RSC Adv. 2018. PMID: 35539110 Free PMC article.
-
Opportunities for Therapeutic Intervention During Machine Perfusion.Curr Transplant Rep. 2017 Jun;4(2):141-148. doi: 10.1007/s40472-017-0144-y. Epub 2017 Apr 19. Curr Transplant Rep. 2017. PMID: 29109929 Free PMC article.
References
-
- Vacanti JP, Kulig KM. Liver cell therapy and tissue engineering for transplantation. Semin Pediatr Surg. 2014;23:150–155. - PubMed
-
- Schlegel A, Kron P, Graf R, Dutkowski P, Clavien PA. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61:1267–1275. - PubMed
-
- Xiang K, Yu H, Yuan J, Li Z. [Feasibility of continuous extracorporeal normothermic liver perfusion using autologous blood: a study in pigs] . Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:223–227. - PubMed
-
- Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, Soltys K, Shiva S, Paranjpe S, Sadowsky D, Barclay D, Zamora R, Stolz D, Demetris A, Michalopoulos G, Marsh JW. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381–394. - PMC - PubMed
LinkOut - more resources
Full Text Sources
