The Immunomodulatory Role of Adjuvants in Vaccines Formulated with the Recombinant Antigens Ov-103 and Ov-RAL-2 against Onchocerca volvulus in Mice
- PMID: 27387453
- PMCID: PMC4936747
- DOI: 10.1371/journal.pntd.0004797
The Immunomodulatory Role of Adjuvants in Vaccines Formulated with the Recombinant Antigens Ov-103 and Ov-RAL-2 against Onchocerca volvulus in Mice
Abstract
Background: In some regions in Africa, elimination of onchocerciasis may be possible with mass drug administration, although there is concern based on several factors that onchocerciasis cannot be eliminated solely through this approach. A vaccine against Onchocerca volvulus would provide a critical tool for the ultimate elimination of this infection. Previous studies have demonstrated that immunization of mice with Ov-103 and Ov-RAL-2, when formulated with alum, induced protective immunity. It was hypothesized that the levels of protective immunity induced with the two recombinant antigens formulated with alum would be improved by formulation with other adjuvants known to enhance different types of antigen-specific immune responses.
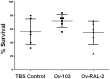
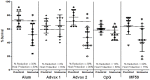
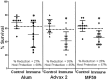
Methodology/ principal findings: Immunizing mice with Ov-103 and Ov-RAL-2 in conjunction with alum, Advax 2 and MF59 induced significant levels of larval killing and host protection. The immune response was biased towards Th2 with all three of the adjuvants, with IgG1 the dominant antibody. Improved larval killing and host protection was observed in mice immunized with co-administered Ov-103 and Ov-RAL-2 in conjunction with each of the three adjuvants as compared to single immunizations. Antigen-specific antibody titers were significantly increased in mice immunized concurrently with the two antigens. Based on chemokine levels, it appears that neutrophils and eosinophils participate in the protective immune response induced by Ov-103, and macrophages and neutrophils participate in immunity induced by Ov-RAL-2.
Conclusions/significance: The mechanism of protective immunity induced by Ov-103 and Ov-RAL-2, with the adjuvants alum, Advax 2 and MF59, appears to be multifactorial with roles for cytokines, chemokines, antibody and specific effector cells. The vaccines developed in this study have the potential of reducing the morbidity associated with onchocerciasis in humans.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: NP is associated with Vaxine Pty Ltd, which has commercial interests in Advax adjuvants. Novartis Vaccines & Diagnostics provided MF59 via a Material Transfer Agreement with the authors and authorized publication of the findings in exchange for access to the data.
Figures

Similar articles
-
Antibody responses against the vaccine antigens Ov-103 and Ov-RAL-2 are associated with protective immunity to Onchocerca volvulus infection in both mice and humans.PLoS Negl Trop Dis. 2019 Sep 16;13(9):e0007730. doi: 10.1371/journal.pntd.0007730. eCollection 2019 Sep. PLoS Negl Trop Dis. 2019. PMID: 31525197 Free PMC article.
-
Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity.Parasite Immunol. 2004 Jan;26(1):53-62. doi: 10.1111/j.0141-9838.2004.00685.x. Parasite Immunol. 2004. PMID: 15198646
-
Predictive immunoinformatics reveal promising safety and anti-onchocerciasis protective immune response profiles to vaccine candidates (Ov-RAL-2 and Ov-103) in anticipation of phase I clinical trials.PLoS One. 2024 Oct 21;19(10):e0312315. doi: 10.1371/journal.pone.0312315. eCollection 2024. PLoS One. 2024. PMID: 39432476 Free PMC article.
-
CD4+-dependent immunity to Onchocerca volvulus third-stage larvae in humans and the mouse vaccination model: common ground and distinctions.Int J Parasitol. 2003 Sep 30;33(11):1161-71. doi: 10.1016/s0020-7519(03)00170-x. Int J Parasitol. 2003. PMID: 13678632 Review.
-
Immunity to Onchocerca spp. in animal hosts.Trends Parasitol. 2002 Apr;18(4):164-71. doi: 10.1016/s1471-4922(02)02245-6. Trends Parasitol. 2002. PMID: 11998704 Review.
Cited by
-
Onchocerca volvulus: The Road from Basic Biology to a Vaccine.Trends Parasitol. 2018 Jan;34(1):64-79. doi: 10.1016/j.pt.2017.08.011. Epub 2017 Sep 22. Trends Parasitol. 2018. PMID: 28958602 Free PMC article. Review.
-
Computational Design and Preliminary Serological Analysis of a Novel Multi-Epitope Vaccine Candidate against Onchocerciasis and Related Filarial Diseases.Pathogens. 2021 Jan 21;10(2):99. doi: 10.3390/pathogens10020099. Pathogens. 2021. PMID: 33494344 Free PMC article.
-
The role of 'omics' in the quest to eliminate human filariasis.PLoS Negl Trop Dis. 2017 Apr 20;11(4):e0005464. doi: 10.1371/journal.pntd.0005464. eCollection 2017 Apr. PLoS Negl Trop Dis. 2017. PMID: 28426656 Free PMC article. Review. No abstract available.
-
Advancing a Human Onchocerciasis Vaccine From Antigen Discovery to Efficacy Studies Against Natural Infection of Cattle With Onchocerca ochengi.Front Cell Infect Microbiol. 2022 Apr 4;12:869039. doi: 10.3389/fcimb.2022.869039. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35444961 Free PMC article. Review.
-
Co-Administration of Adjuvanted Recombinant Ov-103 and Ov-RAL-2 Vaccines Confer Protection against Natural Challenge in A Bovine Onchocerca ochengi Infection Model of Human Onchocerciasis.Vaccines (Basel). 2022 May 27;10(6):861. doi: 10.3390/vaccines10060861. Vaccines (Basel). 2022. PMID: 35746469 Free PMC article.
References
-
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323. Epub 2015/09/15. 10.1016/S0140-6736(15)00128-2 - DOI - PMC - PubMed
-
- Diawara L, Traore MO, Badji A, Bissan Y, Doumbia K, Goita SF, et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS neglected tropical diseases. 2009;3(7):e497 Epub 2009/07/22. 10.1371/journal.pntd.0000497 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

