Porcine Epidemic Diarrhea Virus Infection Inhibits Interferon Signaling by Targeted Degradation of STAT1
- PMID: 27384656
- PMCID: PMC5008104
- DOI: 10.1128/JVI.01091-16
Porcine Epidemic Diarrhea Virus Infection Inhibits Interferon Signaling by Targeted Degradation of STAT1
Abstract
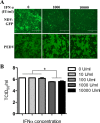
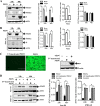
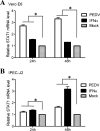
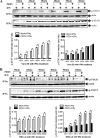
Porcine epidemic diarrhea virus (PEDV) is a worldwide-distributed alphacoronavirus, but the pathogenesis of PEDV infection is not fully characterized. During virus infection, type I interferon (IFN) is a key mediator of innate antiviral responses. Most coronaviruses develop some strategy for at least partially circumventing the IFN response by limiting the production of IFN and by delaying the activation of the IFN response. However, the molecular mechanisms by which PEDV antagonizes the antiviral effects of interferon have not been fully characterized. Especially, how PEDV impacts IFN signaling components has yet to be elucidated. In this study, we observed that PEDV was relatively resistant to treatment with type I IFN. Western blot analysis showed that STAT1 expression was markedly reduced in PEDV-infected cells and that this reduction was not due to inhibition of STAT1 transcription. STAT1 downregulation was blocked by a proteasome inhibitor but not by an autophagy inhibitor, strongly implicating the ubiquitin-proteasome targeting degradation system. Since PEDV infection-induced STAT1 degradation was evident in cells pretreated with the general tyrosine kinase inhibitor, we conclude that STAT1 degradation is independent of the IFN signaling pathway. Furthermore, we report that PEDV-induced STAT1 degradation inhibits IFN-α signal transduction pathways. Pharmacological inhibition of STAT1 degradation rescued the ability of the host to suppress virus replication. Collectively, these data show that PEDV is capable of subverting the type I interferon response by inducing STAT1 degradation.
Importance: In this study, we show that PEDV is resistant to the antiviral effect of IFN. The molecular mechanism is the degradation of STAT1 by PEDV infection in a proteasome-dependent manner. This PEDV infection-induced STAT1 degradation contributes to PEDV replication. Our findings reveal a new mechanism evolved by PEDV to circumvent the host antiviral response.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling.J Virol. 2018 Jan 30;92(4):e01677-17. doi: 10.1128/JVI.01677-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187542 Free PMC article.
-
Innate Immune Evasion of Porcine Epidemic Diarrhea Virus through Degradation of the FBXW7 Protein via the Ubiquitin-Proteasome Pathway.J Virol. 2022 Mar 9;96(5):e0088921. doi: 10.1128/JVI.00889-21. Epub 2021 Sep 8. J Virol. 2022. PMID: 34495699 Free PMC article.
-
Porcine Epidemic Diarrhea Virus Antagonizes Host IFN-λ-Mediated Responses by Tilting Transcription Factor STAT1 toward Acetylation over Phosphorylation To Block Its Activation.mBio. 2023 Jun 27;14(3):e0340822. doi: 10.1128/mbio.03408-22. Epub 2023 Apr 13. mBio. 2023. PMID: 37052505 Free PMC article.
-
Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling.Virus Res. 2016 Dec 2;226:128-141. doi: 10.1016/j.virusres.2016.05.015. Epub 2016 May 19. Virus Res. 2016. PMID: 27212682 Free PMC article. Review.
-
Porcine Epidemic Diarrhea Virus and the Host Innate Immune Response.Pathogens. 2020 May 11;9(5):367. doi: 10.3390/pathogens9050367. Pathogens. 2020. PMID: 32403318 Free PMC article. Review.
Cited by
-
The Dynamic Interface of Viruses with STATs.J Virol. 2020 Oct 27;94(22):e00856-20. doi: 10.1128/JVI.00856-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847860 Free PMC article. Review.
-
Manipulation of Intestinal Antiviral Innate Immunity and Immune Evasion Strategies of Porcine Epidemic Diarrhea Virus.Biomed Res Int. 2019 Nov 3;2019:1862531. doi: 10.1155/2019/1862531. eCollection 2019. Biomed Res Int. 2019. PMID: 31781594 Free PMC article. Review.
-
Porcine epidemic diarrhea virus strain FJzz1 infection induces type I/III IFNs production through RLRs and TLRs-mediated signaling.Front Immunol. 2022 Jul 25;13:984448. doi: 10.3389/fimmu.2022.984448. eCollection 2022. Front Immunol. 2022. PMID: 35958569 Free PMC article.
-
Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling.J Virol. 2018 Jan 30;92(4):e01677-17. doi: 10.1128/JVI.01677-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187542 Free PMC article.
-
CMPK2 is a host restriction factor that inhibits infection of multiple coronaviruses in a cell-intrinsic manner.PLoS Biol. 2023 Mar 17;21(3):e3002039. doi: 10.1371/journal.pbio.3002039. eCollection 2023 Mar. PLoS Biol. 2023. PMID: 36930652 Free PMC article.
References
-
- Temeeyasen G, Srijangwad A, Tripipat T, Tipsombatboon P, Piriyapongsa J, Phoolcharoen W, Chuanasa T, Tantituvanont A, Nilubol D. 2014. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect Genet Evol 21:205–213. doi:10.1016/j.meegid.2013.11.001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

