Efficient Use of a Crude Drug/Herb Library Reveals Ephedra Herb As a Specific Antagonist for TH2-Specific Chemokine Receptors CCR3, CCR4, and CCR8
- PMID: 27376063
- PMCID: PMC4895122
- DOI: 10.3389/fcell.2016.00054
Efficient Use of a Crude Drug/Herb Library Reveals Ephedra Herb As a Specific Antagonist for TH2-Specific Chemokine Receptors CCR3, CCR4, and CCR8
Abstract
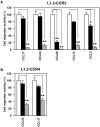
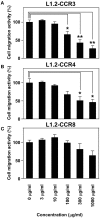
Chemokine receptors CCR3 and CCR4 are preferentially expressed by TH2 cells, mast cells, and/or eosinophils, all of which are involved in the pathogenesis of allergic diseases. Therefore, CCR3 and CCR4 have long been highlighted as potent therapeutic targets for allergic diseases. Japanese traditional herbal medicine Kampo consists of multiple crude drugs/herbs, which further consist of numerous chemical substances. Recent studies have demonstrated that such chemical substances appear to promising sources in the development of novel therapeutic agents. Based on these findings, we hypothesize that Kampo-related crude drugs/herbs would contain chemical substances that inhibit the cell migration mediated by CCR3 and/or CCR4. To test this hypothesis, we screened 80 crude drugs/herbs to identify candidate substances using chemotaxis assay. Among those tested, Ephedra Herb inhibited the chemotaxis mediated by both CCR3 and CCR4, Cornus Fruit inhibited that mediated by CCR3, and Rhubarb inhibited that mediated by CCR4. Furthermore, Ephedra Herb specifically inhibited the chemotaxis mediated by not only CCR3 and CCR4 but CCR8, all of which are selectively expressed by TH2 cells. This result led us to speculate that ephedrine, a major component of Ephedra Herb, would play a central role in the inhibitory effects on the chemotaxis mediated by CCR3, CCR4, and CCR8. However, ephedrine exhibited little effects on the chemotaxis. Therefore, we fractionated Ephedra Herb into four subfractions and examined the inhibitory effects of each subfraction. As the results, ethyl acetate-insoluble fraction exhibited the inhibitory effects on chemotaxis and calcium mobilization mediated by CCR3 and CCR4 most significantly. In contrast, chloroform-soluble fraction exhibited a weak inhibitory effect on the chemotaxis mediated by CCR8. Furthermore, maoto, one of the Kampo formulations containing Ephedra Herb, exhibited the inhibitory effects on the chemotaxis mediated by CCR3, CCR4, and CCR8. Taken together, our data suggest that these crude drugs/herbs might be useful sources to develop new drugs targeting TH2-mediated allergic diseases.
Keywords: CCR3; CCR4; Ephedra Herb; antagonist; chemokine receptor; maoto.
Figures

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Int J Antimicrob Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Epub 2020 Mar 20. Int J Antimicrob Agents. 2020. Retraction in: Int J Antimicrob Agents. 2025 Jan;65(1):107416. doi: 10.1016/j.ijantimicag.2024.107416 PMID: 32205204 Free PMC article. Retracted. Clinical Trial.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
Parathyroid hormone type 1 receptor regulates osteosarcoma K7M2 Cell growth by interacting with angiotensinogen.J Cell Mol Med. 2021 Mar;25(6):2841-2850. doi: 10.1111/jcmm.16314. Epub 2021 Jan 28. J Cell Mol Med. 2021. PMID: 33511766 Free PMC article.
-
A large retroperitoneal lymphatic malformation successfully treated with traditional Japanese Kampo medicine in combination with surgery.Surg Case Rep. 2017 Dec;3(1):80. doi: 10.1186/s40792-017-0358-3. Epub 2017 Jul 17. Surg Case Rep. 2017. PMID: 28718090 Free PMC article.
-
A Case of Suspected Adverse Reactions to Sirolimus in the Treatment of Generalized Lymphatic Anomaly.Case Rep Pediatr. 2019 Apr 30;2019:3101357. doi: 10.1155/2019/3101357. eCollection 2019. Case Rep Pediatr. 2019. PMID: 31183237 Free PMC article.
-
Long-term follow-up for ossification of autologous bone plug and skin sinking after periosteum-preserved burr hole surgery.Surg Neurol Int. 2017 Sep 6;8:204. doi: 10.4103/sni.sni_195_17. eCollection 2017. Surg Neurol Int. 2017. PMID: 28966811 Free PMC article.
References
-
- Forssmann U., Uguccioni M., Loetscher P., Dahinden C. A., Langen H., Thelen M., et al. . (1997). Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 185, 2171–2176. 10.1084/jem.185.12.2171 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

